V体育安卓版 - Phase 1-2 study of docetaxel plus aflibercept in patients with recurrent ovarian, primary peritoneal, or fallopian tube cancer
- PMID: 21992853
- PMCID: PMC3444811
- DOI: 10.1016/S1470-2045(11)70244-3
Phase 1-2 study of docetaxel plus aflibercept in patients with recurrent ovarian, primary peritoneal, or fallopian tube cancer
Abstract
Background: Biologically targeted therapies have been postulated as a viable strategy to improve outcomes for women with ovarian cancer. We assessed the safety, tolerance, pharmacokinetics, relevant circulating and image-derived biomarkers, and clinical activity of combination aflibercept and docetaxel in this population. VSports手机版.
Methods: For the phase 1 (pharmacokinetic) study, eligible patients had measurable, recurrent or persistent epithelial ovarian, primary peritoneal, or fallopian tube carcinoma with a maximum of two prior chemotherapy regimens. Aflibercept was administered intravenously over three dose levels (2, 4, or 6 mg/kg; one dose every 21 days) to identify the maximum tolerated dose for the phase 2 study. Pharmacokinetics were assessed and dynamic imaging was done during a lead-in phase with single-agent aflibercept (cycle 0) and during combination therapy with intravenous docetaxel (75 mg/m(2)). Eligibility for the phase 2 study was the same as for phase 1. Patients were enrolled in a two-stage design and given aflibercept 6 mg/kg intravenously and docetaxel 75 mg/m(2) intravenously, every 3 weeks. The primary endpoint was objective response rate (ORR) as assessed by Response Evaluation Criteria in Solid Tumors version 1. 0. The trial has completed enrolment and all patients are now off study. The trial is registered at ClinicalTrials. gov, number NCT00436501. V体育安卓版.
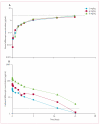
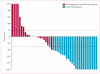
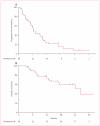
Findings: From the phase 1 study, the recommended phase 2 doses of aflibercept and docetaxel were found to be 6 mg/kg and 75 mg/m(2), respectively. Log-linear pharmacokinetics (for unbound aflibercept) were observed for the three dose levels V体育ios版. No dose-limiting toxicities were noted. 46 evaluable patients were enrolled in the phase 2 trial; 33 were platinum resistant (15 refractory) and 13 were platinum sensitive. The confirmed ORR was 54% (25 of 46; 11 patients had a complete response and 14 had a partial response). Grade 3-4 toxicities observed in more than two patients (5%) were: neutropenia in 37 patients (80%); leucopenia in 25 patients (54%); fatigue in 23 patients (50%); dyspnoea in ten patients (22%); and stomatitis in three patients (7%). Adverse events specifically associated with aflibercept were grade 1-2 hypertension in five patients (11%), and grade 2 proteinuria in one patient (2%). .
Interpretation: Combination aflibercept plus docetaxel can be safely administered at the dose and schedule reported here, and is associated with substantial antitumour activity VSports最新版本. These findings suggest that further clinical development of this combination in ovarian cancer is warranted. .
Funding: US National Cancer Institute, US Department of Defense, Sanofi-Aventis, Gynecologic Cancer Foundation, Marcus Foundation, and the Commonwealth Foundation V体育平台登录. .
Copyright © 2011 Elsevier Ltd VSports注册入口. All rights reserved. .
Figures
VSports app下载 - Comment in
-
VEGF inhibitors and advanced ovarian cancer. (V体育安卓版)Lancet Oncol. 2011 Nov;12(12):1082-3. doi: 10.1016/S1470-2045(11)70269-8. Epub 2011 Oct 10. Lancet Oncol. 2011. PMID: 21992854 No abstract available.
References
-
- Tew WP, Colombo N, Ray-Coquard I, et al. VEGF-Trap for patients (pts) with recurrent platinum-resistant epithelial ovarian cancer (EOC): preliminary results of a randomized, multicenter phase II study. Proc Am Soc Clin Oncol. 2007;25 abstr 5508.
Publication types
- "V体育平台登录" Actions
- "VSports app下载" Actions
MeSH terms
- "V体育平台登录" Actions
- "V体育官网入口" Actions
- "VSports" Actions
- "VSports注册入口" Actions
- Actions (V体育ios版)
- VSports - Actions
- V体育官网 - Actions
- "VSports最新版本" Actions
- "V体育平台登录" Actions
- "V体育2025版" Actions
- Actions (V体育安卓版)
- Actions (V体育官网)
"V体育ios版" Substances
- VSports注册入口 - Actions
- VSports最新版本 - Actions
Associated data (VSports最新版本)
Grants and funding
LinkOut - more resources
Full Text Sources
V体育ios版 - Other Literature Sources
"VSports最新版本" Medical

