Activation of p38 MAPK in CD4 T cells controls IL-17 production and autoimmune encephalomyelitis
- PMID: 21791428
- PMCID: PMC3179398
- DOI: 10.1182/blood-2011-02-336552
Activation of p38 MAPK in CD4 T cells controls IL-17 production and autoimmune encephalomyelitis
Abstract
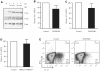
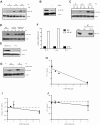
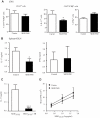
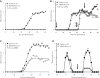
Although several transcription factors have been shown to be critical for the induction and maintenance of IL-17 expression by CD4 Th cells, less is known about the role of nontranscriptional mechanisms. Here we show that the p38 MAPK signaling pathway is essential for in vitro and in vivo IL-17 production by regulating IL-17 synthesis in CD4 T cells through the activation of the eukaryotic translation initiation factor 4E/MAPK-interacting kinase (eIF-4E/MNK) pathway. We also show that p38 MAPK activation is required for the development and progression of both chronic and relapsing-remitting forms of experimental allergic encephalomyelitis (EAE), the principal autoimmune model of multiple sclerosis. Furthermore, we show that regulation of p38 MAPK activity specifically in T cells is sufficient to modulate EAE severity. Thus, mechanisms other than the regulation of gene expression also contribute to Th17 cell effector functions and, potentially, to the pathogenesis of other Th17 cell-mediated diseases. VSports手机版.
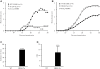
Figures

V体育2025版 - References
-
- Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112(5):1557–1569. - VSports app下载 - PMC - PubMed
-
- Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8(5):337–348. - PubMed
-
- Awasthi A, Kuchroo VK. Th17 cells: from precursors to players in inflammation and infection. Int Immunol. 2009;21(5):489–498. - PMC (V体育安卓版) - PubMed
Publication types (V体育安卓版)
- Actions (VSports)
"VSports app下载" MeSH terms
- Actions (V体育2025版)
- V体育2025版 - Actions
- Actions (VSports在线直播)
- "VSports在线直播" Actions
- "V体育ios版" Actions
- "VSports注册入口" Actions
- "VSports注册入口" Actions
- "V体育ios版" Actions
- VSports最新版本 - Actions
- Actions (VSports app下载)
- V体育官网 - Actions
- Actions (V体育ios版)
- VSports在线直播 - Actions
- V体育ios版 - Actions
- VSports手机版 - Actions
- "V体育官网" Actions
- Actions (V体育平台登录)
- VSports注册入口 - Actions
- "V体育安卓版" Actions
- Actions (VSports app下载)
- V体育官网入口 - Actions
- Actions (V体育官网)
"V体育官网入口" Substances
- Actions (V体育官网入口)
- "VSports注册入口" Actions
Grants and funding (V体育官网)
- NS060901/NS/NINDS NIH HHS/United States
- R01 NS036526/NS/NINDS NIH HHS/United States
- AI041747/AI/NIAID NIH HHS/United States (VSports注册入口)
- R01 NS060901/NS/NINDS NIH HHS/United States
- VSports在线直播 - AI054154/AI/NIAID NIH HHS/United States
- T32 AI055402/AI/NIAID NIH HHS/United States
- R01 AI051454/AI/NIAID NIH HHS/United States
- "VSports最新版本" R01 AI041747/AI/NIAID NIH HHS/United States
- V体育官网 - R21 NS076200/NS/NINDS NIH HHS/United States
- NS036526/NS/NINDS NIH HHS/United States
- P20 RR021905/RR/NCRR NIH HHS/United States (V体育安卓版)
- R01 NS069628/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

