Competition between PARP-1 and Ku70 control the decision between high-fidelity and mutagenic DNA repair
- PMID: 21256093
- PMCID: PMC4079052
- DOI: 10.1016/j.dnarep.2010.12.005 (VSports手机版)
Competition between PARP-1 and Ku70 control the decision between high-fidelity and mutagenic DNA repair
Abstract
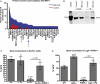
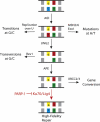
Affinity maturation of antibodies requires a unique process of targeted mutation that allows changes to accumulate in the antibody genes while the rest of the genome is protected from off-target mutations that can be oncogenic. This targeting requires that the same deamination event be repaired either by a mutagenic or a high-fidelity pathway depending on the genomic location VSports手机版. We have previously shown that the BRCT domain of the DNA-damage sensor PARP-1 is required for mutagenic repair occurring in the context of IgH and IgL diversification in the chicken B cell line DT40. Here we show that immunoprecipitation of the BRCT domain of PARP-1 pulls down Ku70 and the DNA-PK complex although the BRCT domain of PARP-1 does not bind DNA, suggesting that this interaction is not DNA dependent. Through sequencing the IgL variable region in PARP-1(-/-) cells that also lack Ku70 or Lig4, we show that Ku70 or Lig4 deficiency restores GCV to PARP-1(-/-) cells and conclude that the mechanism by which PARP-1 is promoting mutagenic repair is by inhibiting high-fidelity repair which would otherwise be mediated by Ku70 and Lig4. .
Copyright © 2010 Elsevier B V体育安卓版. V. All rights reserved. .
Figures

References
-
- Fugmann SD, Lee AI, Shockett PE, Villey IJ, Schatz DG. The RAG proteins and V(D)J recombination: complexes, ends, and transposition. Annu. Rev. Immunol. 2000;18:495–527. - PubMed
-
- Taccioli GE, Rathbun G, Oltz E, Stamato T, Jeggo PA, Alt FW. Impairment of V(D)J recombination in double-strand break repair mutants. Science. 1993;260:207–210. - "V体育2025版" PubMed
-
- Grawunder U, Zimmer D, Fugmann S, Schwarz K, Lieber MR. DNA ligase IV is essential for V(D)J recombination and DNA double-strand break repair in human precursor lymphocytes. Mol. Cell. 1998;2:477–484. - PubMed (V体育官网)
-
- Craig R, Beavis RC. TANDEM: matching proteins with tandem mass spectra. Bioinformatics. 2004;20:1466–1467. - PubMed
Publication types
- Actions (VSports注册入口)
- Actions (V体育官网入口)
MeSH terms
- Actions (VSports在线直播)
- "V体育安卓版" Actions
- "V体育官网" Actions
- "VSports app下载" Actions
- Actions (V体育2025版)
- VSports - Actions
- Actions (VSports app下载)
- "V体育官网" Actions
- VSports最新版本 - Actions
- Actions (VSports注册入口)
- Actions (V体育安卓版)
Substances
- Actions (VSports最新版本)
- "VSports app下载" Actions
- V体育ios版 - Actions
- Actions (VSports注册入口)
- VSports - Actions
- Actions (V体育2025版)
- Actions (V体育安卓版)
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
VSports在线直播 - Miscellaneous

