Design, synthesis, and biological evaluations of 2,5-diaryl-2,3-dihydro-1,3,4-oxadiazoline analogs of combretastatin-A4
- PMID: 19894742
- PMCID: PMC2810428
- DOI: 10.1021/jm901268n
Design, synthesis, and biological evaluations of 2,5-diaryl-2,3-dihydro-1,3,4-oxadiazoline analogs of combretastatin-A4
"VSports" Abstract
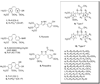
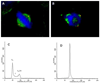
A total of 24 novel 2,5-diaryl-1,3,4-oxadiazoline analogs of combretastatin A-4 (CA-4, 1) were designed, synthesized, and evaluated for biological activities. The compounds represent two structural classes; the Type I class has three methoxy groups on the A ring and the Type II class has a single methoxy group on the A ring. Biological evaluations demonstrate that multiple structural features control the biological potency. Four of the compounds, 2-(3'-bromophenyl)-5-(3'',4'',5''-trimethoxyphenyl)-2-acetyl-2,3-dihydro-1,3,4-oxadiazoline (9l), 2-(2',5'-dimethoxyphenyl)-5-(3''-methoxyphenyl)-2-acetyl-2,3-dihydro-1,3,4-oxadiazoline (10h), 2-(3',4',5'-trimethoxyphenyl)-5-(3''-methoxyphenyl)-2-acetyl-2,3-dihydro-1,3,4-oxadiazoline (10i), and 2-(3',5'-dimethoxyphenyl)-5-(3''-methoxyphenyl)-2-acetyl-2,3-dihydro-1,3,4-oxadiazoline (10j), have potent antiproliferative activities against multiple cancer cell lines. Mechanistic studies indicate that they retain the microtubule disrupting effects of compound 1, including microtubule loss, the formation of aberrant mitotic spindles, and mitotic arrest. Compound 10i inhibits purified tubulin polymerization and circumvents drug resistance mediated by P-glycoprotein and betaIII tubulin expression. The oxadiazoline analog 10i is a promising lead candidate worthy of further investigation VSports手机版. .
"VSports注册入口" Figures




References
-
- Pettit GR, Singh SB, Niven ML, Hamel E, Schmidt JM. Isolation, structure, and synthesis of combretastatins A-1 and B-1, potent new inhibitors of microtubule assembly derived from Combretum caffrum. J. Nat. Prod. 1987;50:119–131. - VSports在线直播 - PubMed
-
- Pettit GR, Cragg GM, Singh SB. Antineoplastic agents, 122. Constituents of Combretum caffrum. J. Nat. Prod. 1987;50:386–391. - PubMed
-
- Pettit GR, Singh SB, Hamel E, Lin CM, Alberts DS, Garcia-Kendall D. Isolation and structure of the strong cell growth and tubulin inhibitor combretastatin A-4. Experientia. 1989;45:209–211. - "VSports app下载" PubMed
-
- Lin CM, Singh SB, Chu PS, Dempcy RO, Schmidt JM, Pettit GR, Hamel E. Interactions of tubulin with potent natural and synthetic analogs of the antimitotic agent combretastatin: a structure-activity study. Mol. Pharmacol. 1988;34:200–208. - VSports - PubMed
-
- Dorr RT, Dvorakova K, Snead K, Alberts DS, Salmon SE, Pettit GR. Antitumor activity of combretastatin-A4 phosphate, a natural product tubulin inhibitor. Invest. New Drugs. 1996;14:131–137. - PubMed (V体育2025版)
VSports手机版 - Publication types
- Actions (VSports app下载)
MeSH terms (VSports注册入口)
- Actions (V体育2025版)
- VSports手机版 - Actions
- Actions (VSports)
- Actions (V体育平台登录)
- Actions (VSports手机版)
- "V体育平台登录" Actions
- "V体育ios版" Actions
Substances
- Actions (V体育平台登录)
- Actions (V体育ios版)
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

