Development and optimization of a cell-based assay for the selection of synthetic compounds that potentiate bone morphogenetic protein-2 activity
- PMID: 19862690
- PMCID: PMC2858640
- DOI: V体育官网 - 10.1002/cbf.1615
Development and optimization of a cell-based assay for the selection of synthetic compounds that potentiate bone morphogenetic protein-2 activity (V体育安卓版)
Abstract (V体育2025版)
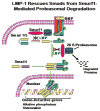
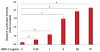
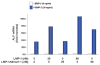
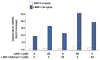
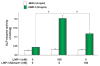
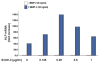
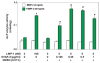
The requirement of large amounts of the recombinant human bone morphogenetic protein-2 (BMP-2) produces a huge translational barrier for its routine clinical use due to high cost. This leads to an urgent need to develop alternative methods to lower costs and/or increase efficacies for using BMP-2. In this study, we describe the development and optimization of a cell-based assay that is sensitive, reproducible, and reliable in identifying reagents that potentiate the effects of BMP-2 in inducing transdifferentiation of C2C12 myoblasts into the osteoblastic phenotype. The assay is based on a BMP-responsive Smad1-driven luciferase reporter gene. LIM mineralization protein-1 (LMP-1) is a novel intracellular LIM domain protein that has been shown by our group to enhance cellular responsiveness to BMP-2. Our previous report elucidated that the binding of LMP-1 with the WW2 domain in Smad ubiquitin regulatory factor-1 (Smurf1) rescues the osteogenic Smads from degradation. Here, using the optimized cell-based assay, we first evaluated the activity of the recombinantly prepared proteins, LMP-1, and its mutant (LMP-1DeltaSmurf1) that lacks the Smurf1-WW2 domain-binding motif. Both the wild type and the mutant proteins were engineered to contain an 11-amino acid HIV-TAT protein derived membrane transduction domain to aid the cellular delivery of recombinant proteins. The cell-based reporter assay confirmed that LMP-1 potentiates the BMP-induced stimulation of C2C12 cells towards the osteoblastic phenotype. The potentiating effect of LMP-1 was significantly reduced when a specific-motif known to interact with Smurf1 was mutated. We validated the results obtained in the reporter assay by also monitoring the expression of mRNA for osteocalcin and alkaline phosphatase (ALP) which is widely accepted osteoblast differentiation marker genes. Finally, we provide further confirmation of our results by measuring the activity of alkaline phosphatase in support of the accuracy and reliability of our cell-based assay. Direct delivery of synthesized protein can be limited by high cost, instability or inadequate post-translational modifications. Thus, there would be a clear benefit for a low cost, cell penetrable chemical compound. We successfully used our gene expression-based assay to choose an active compound from a select group of compounds that were identified by computational screenings as the most likely candidates for mimicking the function of LMP-1 VSports手机版. Among them, we selected SVAK-3, a compound that showed a dose-dependent potentiation of BMP-2 activity in inducing osteoblastic differentiation of C2C12 cells. We show that either the full length LMP-1 protein or its potential mimetic compound consistently exhibit similar potentiation of BMP-2 activity even when multiple markers of the osteoblastic phenotype were parallely monitored. .
Figures


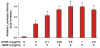
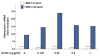
References
-
- Cheng H, Jiang W, Phillips FM, et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs) J Bone Joint Surg Am. 2003;85-A:1544–1552. - PubMed
-
- Cheng SL, Shao JS, Charlton-Kachigian N, Loewy AP, Towler DA. MSX2 promotes osteogenesis and suppresses adipogenic differentiation of multipotent mesenchymal progenitors. J Biol Chem. 2003;278:45969–45977. - "VSports注册入口" PubMed
-
- Peng Y, Kang Q, Cheng H, et al. Transcriptional characterization of bone morphogenetic proteins (BMPs)-mediated osteogenic signaling. J Cell Biochem. 2003;90:1149–1165. - PubMed
-
- von Bubnoff A, Cho KW. Intracellular BMP signaling regulation in vertebrates: pathway or network? Dev Biol. 2001;239:1–14. - PubMed
"V体育ios版" Publication types
- "VSports最新版本" Actions
MeSH terms
- "VSports" Actions
- "V体育平台登录" Actions
- "VSports在线直播" Actions
- "V体育ios版" Actions
- "V体育安卓版" Actions
Substances
- V体育官网 - Actions
- V体育官网 - Actions
- "VSports在线直播" Actions
- "VSports最新版本" Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

