Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer (V体育ios版)
- PMID: 18725988
- PMCID: PMC2518073
- DOI: VSports注册入口 - 10.1172/JCI34739
VSports注册入口 - Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer
Abstract
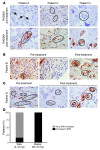
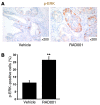
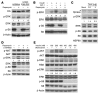
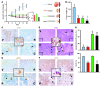
Numerous studies have established a causal link between aberrant mammalian target of rapamycin (mTOR) activation and tumorigenesis, indicating that mTOR inhibition may have therapeutic potential VSports手机版. In this study, we show that rapamycin and its analogs activate the MAPK pathway in human cancer, in what represents a novel mTORC1-MAPK feedback loop. We found that tumor samples from patients with biopsy-accessible solid tumors of advanced disease treated with RAD001, a rapamycin derivative, showed an administration schedule-dependent increase in activation of the MAPK pathway. RAD001 treatment also led to MAPK activation in a mouse model of prostate cancer. We further show that rapamycin-induced MAPK activation occurs in both normal cells and cancer cells lines and that this feedback loop depends on an S6K-PI3K-Ras pathway. Significantly, pharmacological inhibition of the MAPK pathway enhanced the antitumoral effect of mTORC1 inhibition by rapamycin in cancer cells in vitro and in a xenograft mouse model. Taken together, our findings identify MAPK activation as a consequence of mTORC1 inhibition and underscore the potential of a combined therapeutic approach with mTORC1 and MAPK inhibitors, currently employed as single agents in the clinic, for the treatment of human cancers. .
Figures




Comment in
- J Clin Invest. 118:3003.
References
Publication types
MeSH terms (V体育2025版)
- V体育官网入口 - Actions
- Actions (VSports)
- Actions (VSports手机版)
- V体育2025版 - Actions
- VSports最新版本 - Actions
- "VSports" Actions
- VSports - Actions
- "VSports手机版" Actions
- V体育官网 - Actions
- Actions (V体育ios版)
- "V体育官网入口" Actions
- "V体育官网入口" Actions
Substances
- "V体育官网入口" Actions
- Actions (VSports注册入口)
- VSports app下载 - Actions
- VSports注册入口 - Actions
- "V体育官网" Actions
- "VSports" Actions
Grants and funding
V体育2025版 - LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
VSports在线直播 - Miscellaneous

