Role of fibroblast growth factor type 1 and 2 in carbon tetrachloride-induced hepatic injury and fibrogenesis
- PMID: 14507672
- PMCID: PMC1868310
- DOI: "V体育官网" 10.1016/S0002-9440(10)63522-5
"VSports在线直播" Role of fibroblast growth factor type 1 and 2 in carbon tetrachloride-induced hepatic injury and fibrogenesis
Abstract
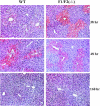
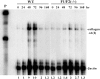
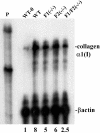
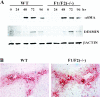
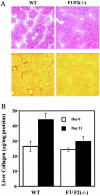
Genomic ablation of hepatocyte-specific fibroblast growth factor receptor (FGFR)4 in mice revealed a role of FGF signaling in cholesterol and bile acid metabolism and hepatolobular restoration in response to injury without effect on liver development or hepatocyte proliferation. Although the potential role of all 23 FGF polypeptides in the liver is still unclear, the most widely studied prototypes, FGF1 and FGF2, are present and have been implicated in liver cell growth and function in vitro VSports手机版. To determine whether FGF1 and FGF2 play a role in response to injury and fibrosis, we examined the impact of both acute and chronic exposure to carbon tetrachloride (CCl(4)) in the livers of FGF1- and FGF2-deficient mice. After acute CCl(4) exposure, FGF1(-/-)FGF2(-/-) mice exhibited an accelerated release of serum alanine aminotransferase similar to FGFR4 deficiency, but no effect on overall hepatolobular restoration or bile acid metabolism. FGF1(-/-)FGF2(-/-) mice exhibited a normal increase in alpha-smooth muscle actin and desmin associated with activation and migration of hepatic stellate cells to damage, but a reduced level of hepatic stellate cell-derived matrix collagen alpha1(I) synthesis. Liver fibrosis resulting from chronic CCl(4) exposure was markedly decreased in the livers of FGF1/FGF2-deficient mice. These results suggest an agonist role for FGF1 and FGF2 in specifically insult-induced liver matrix deposition and hepatic fibrogenesis and a potential target for the prevention of hepatic fibrosis. .
Figures

References
-
- McKeehan WL, Wang F, Kan M: The heparan sulfate-fibroblast growth factor family: diversity of structure and function. Prog Nucleic Acid Res Mol Biol 1998, 59:135-176 - "VSports注册入口" PubMed
-
- Powers CJ, McLeskey SW, Wellstein A: Fibroblast growth factors, their receptors and signaling. Endocr Relat Cancer 2000, 7:165-197 - PubMed (V体育2025版)
-
- Abraham JA, Mergia A, Whang JL, Tumolo A, Friedman J, Hjerrild KA, Gospodarowicz D, Fiddes JC: Nucleotide sequence of a bovine clone encoding the angiogenic protein, basic fibroblast growth factor. Science 1986, 233:545-548 - V体育官网入口 - PubMed
-
- Jaye M, Howk R, Burgess W, Ricca GA, Chiu IM, Ravera MW, O’Brien SJ, Modi WS, Maciag T, Drohan WN: Human endothelial cell growth factor: cloning, nucleotide sequence, and chromosome localization. Science 1986, 233:541-545 - PubMed
V体育2025版 - Publication types
MeSH terms
- Actions (VSports app下载)
- Actions (V体育平台登录)
- V体育官网 - Actions
- Actions (V体育安卓版)
- "V体育安卓版" Actions
- "VSports手机版" Actions
- "V体育ios版" Actions
- "VSports app下载" Actions
- "V体育官网" Actions
- Actions (VSports最新版本)
- VSports - Actions
Substances
- Actions (V体育安卓版)
- VSports手机版 - Actions
- VSports注册入口 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
"VSports" Miscellaneous

