V体育ios版 - DDR2 receptor promotes MMP-2-mediated proliferation and invasion by hepatic stellate cells
- PMID: 11696582
- PMCID: PMC209436
- DOI: V体育平台登录 - 10.1172/JCI12373
DDR2 receptor promotes MMP-2-mediated proliferation and invasion by hepatic stellate cells
Abstract
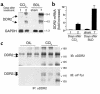
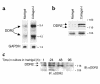
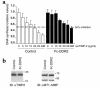
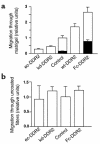
Type I collagen provokes activation of hepatic stellate cells during liver injury through mechanisms that have been unclear VSports手机版. Here, we tested the role of the discoidin domain tyrosine kinase receptor 2 (DDR2), which signals in response to type I collagen, in this pathway. DDR2 mRNA and protein are induced in stellate cells activated by primary culture or in vivo during liver injury. The receptor becomes tyrosine phosphorylated in response to either endogenous or exogenous type I collagen, whereas its expression is downregulated during cellular quiescence induced by growth on Matrigel. We developed stellate cell lines stably overexpressing either wild-type DDR2, a constitutively active chimeric DDR2 receptor (Fc-DDR2), a truncated receptor expressing the extracellular domain, or a kinase-dead DDR2 Cells overexpressing DDR2 showed enhanced proliferation and invasion through Matrigel, activities that were directly related to increased expression of active matrix metalloproteinase 2 (MMP-2). These data show that DDR2 is induced during stellate cell activation and implicate the phosphorylated receptor as a mediator of MMP-2 release and growth stimulation in response to type I collagen. Moreover, type I collagen-dependent upregulation of DDR2 expression establishes a positive feedback loop in activated stellate cells, leading to further proliferation and enhanced invasive activity. .
Figures


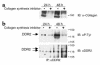
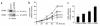
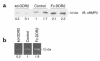
References
-
- Friedman SL. Molecular regulation of hepatic fibrosis; an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–2250. - PubMed
-
- Olaso E, Friedman SL. Molecular regulation of hepatic fibrogenesis. J Hepatol. 1998;29:836–847. - PubMed
-
- Friedman SL, Roll FJ, Boyles J, Arenson DM, Bissell DM. Maintenance of differentiated phenotype of cultured rat hepatic lipocytes by basement membrane matrix. J Biol Chem. 1989;264:10756–10762. - PubMed
-
- Butler GS, et al. The TIMP2 membrane type 1 metalloproteinase receptor regulates the concentration and efficient activation of progelatinase A. J Biol Chem. 1998;273:871–880. - PubMed
Publication types
- "V体育2025版" Actions
- VSports最新版本 - Actions
MeSH terms
- V体育官网入口 - Actions
- "VSports注册入口" Actions
- V体育ios版 - Actions
- V体育ios版 - Actions
- Actions (V体育ios版)
- Actions (VSports手机版)
- "VSports在线直播" Actions
- V体育官网 - Actions
- Actions (V体育官网)
- V体育安卓版 - Actions
- V体育平台登录 - Actions
- Actions (VSports app下载)
- Actions (V体育2025版)
V体育官网 - Substances
- VSports在线直播 - Actions
- "V体育官网入口" Actions
- "V体育ios版" Actions
Grants and funding
LinkOut - more resources
Full Text Sources
"V体育官网入口" Other Literature Sources
Miscellaneous

