Induction of terminal differentiation by constitutive activation of p38 MAP kinase in human rhabdomyosarcoma cells
- PMID: 10716945
- PMCID: PMC316418
Induction of terminal differentiation by constitutive activation of p38 MAP kinase in human rhabdomyosarcoma cells (VSports app下载)
Abstract
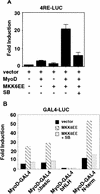
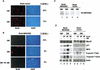
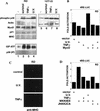
MyoD inhibits cell proliferation and promotes muscle differentiation. A paradoxical feature of rhabdomyosarcoma (RMS), a tumor arising from muscle precursors, is the block of the differentiation program and the deregulated proliferation despite MyoD expression. A deficiency in RMS of a factor required for MyoD activity has been implicated by previous studies. We report here that p38 MAP kinase (MAPK) activation, which is essential for muscle differentiation, is deficient in RMS cells VSports手机版. Enforced induction of p38 MAPK by an activated MAPK kinase 6 (MKK6EE) restored MyoD function and enhanced MEF2 activity in RMS deficient for p38 MAPK activation, leading to growth arrest and terminal differentiation. Stress and cytokines could activate the p38 MAPK in RMS cells, however, these stimuli did not promote differentiation, possibly because they activated p38 MAPK only transiently and they also activated JNK, which could antagonize differentiation. Thus, the selective and sustained p38 MAPK activation, which is distinct from the stress-activated response, is required for differentiation and can be disrupted in human tumors. .
Figures
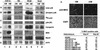



References
-
- Alemà S, Tatò F. Oncogenes and muscle differentiation: Multiple mechanism of interference. Semin Cancer Biol. 1994;5:147–156. - PubMed
-
- Arndt CAS, Crist WM. Medical progress: Common musculoskeletal tumors of childhood and adolescence. N Engl J Med. 1999;341:342–353. - "V体育官网" PubMed
-
- Arnold H-H, Winter B. Muscle differentiation: More complexity to the network of myogenic regulators. Curr Opin Genet Dev. 1998;8:539–544. - "VSports app下载" PubMed
-
- Bengal E, Ransone L, Scharfmann R, Dwarki VJ, Tapscott SJ, Weintraub H, Verma IM. Functional antagonism between cJun and MyoD proteins: a direct physical association. Cell. 1992;68:507–519. - PubMed
-
- Black BL, Molkentin JD, Olson E. Multiples roles for the MyoD basic region in transmission of transcriptional activation signals and interaction with Mef2. Mol Cell Biol. 1998;18:69–77. - V体育安卓版 - PMC - PubMed
V体育2025版 - Publication types
- Actions (VSports)
MeSH terms
- "V体育官网入口" Actions
- Actions (VSports最新版本)
- Actions (V体育平台登录)
- Actions (VSports注册入口)
- V体育官网 - Actions
- "VSports在线直播" Actions
- V体育安卓版 - Actions
- "VSports最新版本" Actions
- "VSports手机版" Actions
Substances
- VSports - Actions
- "V体育2025版" Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
