Bcl-2-mediated drug resistance: inhibition of apoptosis by blocking nuclear factor of activated T lymphocytes (NFAT)-induced Fas ligand transcription
- PMID: 10432288
- PMCID: PMC2195578
- DOI: "V体育平台登录" 10.1084/jem.190.2.253
Bcl-2-mediated drug resistance: inhibition of apoptosis by blocking nuclear factor of activated T lymphocytes (NFAT)-induced Fas ligand transcription
Abstract
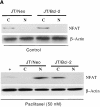
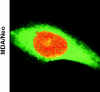
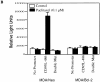
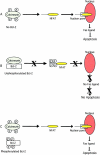
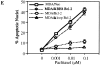
Bcl-2 inhibits apoptosis induced by a variety of stimuli, including chemotherapy drugs and glucocorticoids. It is generally accepted that Bcl-2 exerts its antiapoptotic effects mainly by dimerizing with proapoptotic members of the Bcl-2 family such as Bax and Bad. However, the mechanism of the antiapoptotic effects is unclear VSports手机版. Paclitaxel and other drugs that disturb microtubule dynamics kill cells in a Fas/Fas ligand (FasL)-dependent manner; antibody to FasL inhibits paclitaxel-induced apoptosis. We have found that Bcl-2 overexpression leads to the prevention of chemotherapy (paclitaxel)-induced expression of FasL and blocks paclitaxel-induced apoptosis. The mechanism of this effect is that Bcl-2 prevents the nuclear translocation of NFAT (nuclear factor of activated T lymphocytes, a transcription factor activated by microtubule damage) by binding and sequestering calcineurin, a calcium-dependent phosphatase that must dephosphorylate NFAT to move to the nucleus. Without NFAT nuclear translocation, the FasL gene is not transcribed. Thus, it appears that paclitaxel and other drugs that disturb microtubule function kill cells at least in part through the induction of FasL. Furthermore, Bcl-2 antagonizes drug-induced apoptosis by inhibiting calcineurin activation, blocking NFAT nuclear translocation, and preventing FasL expression. The effects of Bcl-2 can be overcome, at least partially, through phosphorylation of Bcl-2. Phosphorylated Bcl-2 cannot bind calcineurin, and NFAT activation, FasL expression, and apoptosis can occur after Bcl-2 phosphorylation. .
Figures





















References
-
- Watanabe-Fukunaga R., Brannan C.I., Copeland N.G., Jenkins N.A., Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–317. - "V体育安卓版" PubMed
-
- Leithauser F., Dhein J., Mechtersheimer G., Koretz K., Bruderlein S., Henne C., Schmidt A., Debatin K.M., Krammer P.H., Moller P. Constitutive and induced expression of APO-1, a new member of the nerve growth factor/tumor necrosis factor receptor superfamily, in normal and neoplastic cells. Lab. Invest. 1993;69:415–429. - PubMed
-
- French L.E., Hahne M., Viard I., Radlgruber G., Zanone R., Becker K., Muller C., Tschopp J. Fas and Fas ligand in embryos and adult miceligand expression in several immune-privileged tissues and coexpression in adult tissues characterized by apoptotic cell turnover. J. Cell Biol. 1996;133:335–343. - PMC - PubMed
-
- Rouvier E., Luciani M.F., Golstein P. Fas involvement in Ca2+-independent T cell–mediated cytotoxicity. J. Exp. Med. 1993;177:195–200. - PMC (V体育官网入口) - PubMed
Publication types
MeSH terms
- Actions (V体育官网入口)
- "VSports在线直播" Actions
- "VSports" Actions
- Actions (VSports手机版)
- "VSports app下载" Actions
- "V体育平台登录" Actions
- V体育2025版 - Actions
- VSports最新版本 - Actions
- "V体育安卓版" Actions
- "VSports手机版" Actions
- VSports注册入口 - Actions
Substances
- Actions (VSports手机版)
- VSports注册入口 - Actions
- "VSports app下载" Actions
- "VSports" Actions
"VSports" LinkOut - more resources
Full Text Sources
Research Materials (V体育平台登录)
VSports在线直播 - Miscellaneous

