"VSports最新版本" The regulation of reactive oxygen species production during programmed cell death
- PMID: 9628898
- PMCID: PMC2132785
- DOI: V体育平台登录 - 10.1083/jcb.141.6.1423
"V体育2025版" The regulation of reactive oxygen species production during programmed cell death
Abstract
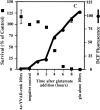
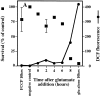
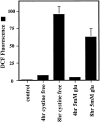
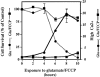
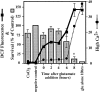
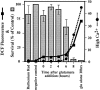
Reactive oxygen species (ROS) are thought to be involved in many forms of programmed cell death. The role of ROS in cell death caused by oxidative glutamate toxicity was studied in an immortalized mouse hippocampal cell line (HT22). The causal relationship between ROS production and glutathione (GSH) levels, gene expression, caspase activity, and cytosolic Ca2+ concentration was examined. An initial 5-10-fold increase in ROS after glutamate addition is temporally correlated with GSH depletion. This early increase is followed by an explosive burst of ROS production to 200-400-fold above control values. The source of this burst is the mitochondrial electron transport chain, while only 5-10% of the maximum ROS production is caused by GSH depletion. Macromolecular synthesis inhibitors as well as Ac-YVAD-cmk, an interleukin 1beta-converting enzyme protease inhibitor, block the late burst of ROS production and protect HT22 cells from glutamate toxicity when added early in the death program VSports手机版. Inhibition of intracellular Ca2+ cycling and the influx of extracellular Ca2+ also blocks maximum ROS production and protects the cells. The conclusion is that GSH depletion is not sufficient to cause the maximal mitochondrial ROS production, and that there is an early requirement for protease activation, changes in gene expression, and a late requirement for Ca2+ mobilization. .
Figures




References
-
- Abe J, Takahashi M, Ishida M, Lee J-D, Berk BC. c-Src is required for oxidative stress-mediated activation of big mitogen-activated protein kinase 1 (BMK1) J Biol Chem. 1997;272:20389–20394. - PubMed
-
- Bass DA, Parce JW, Dechatelet LR, Szejda P, Seeds MC, Thomas M. Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J Immunol. 1983;130:1910–1917. - PubMed (VSports注册入口)
-
- Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–716. - "VSports在线直播" PMC - PubMed
-
- Boveris A, Oshino N, Chance B. The cellular production of hydrogen peroxide. Biochem J. 1972;128:617–630. - PMC (V体育官网) - PubMed
Publication types
- Actions (V体育ios版)
MeSH terms
- Actions (VSports注册入口)
- V体育安卓版 - Actions
- Actions (VSports手机版)
- "VSports在线直播" Actions
- Actions (V体育2025版)
- VSports注册入口 - Actions
- Actions (V体育官网入口)
- "VSports app下载" Actions
Substances
- V体育安卓版 - Actions
- "VSports app下载" Actions
VSports最新版本 - Grants and funding
LinkOut - more resources
"VSports" Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous (VSports最新版本)

