RecQ helicase, in concert with RecA and SSB proteins, initiates and disrupts DNA recombination (V体育安卓版)
- PMID: 9553043
- PMCID: "V体育2025版" PMC316708
- DOI: VSports - 10.1101/gad.12.8.1134
RecQ helicase, in concert with RecA and SSB proteins, initiates and disrupts DNA recombination
V体育ios版 - Abstract
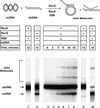
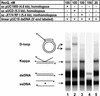
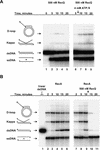
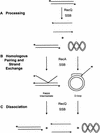
RecQ helicase is important to homologous recombination and DNA repair in Escherichia coli. We demonstrate that RecQ helicase, in conjunction with RecA and SSB proteins, can initiate recombination events in vitro. In addition, RecQ protein is capable of unwinding a wide variety of DNA substrates, including joint molecules formed by RecA protein VSports手机版. These data are consistent with RecQ helicase assuming two roles in the cell; it can be (1) an initiator of homologous recombination, or (2) a disrupter of joint molecules formed by aberrant recombination. These findings also shed light on the function of the eukaryotic homologs of RecQ helicase, the Sgs1, Blm, and Wrn helicases. .
Figures
References (V体育2025版)
-
- Anderson DG, Kowalczykowski SC. The recombination hot spot χ is a regulatory element that switches the polarity of DNA degradation by the RecBCD enzyme. Genes & Dev. 1997a;11:571–581. - PubMed
-
- ————— The translocating RecBCD enzyme stimulates recombination by directing RecA protein onto ssDNA in a chi-regulated manner. Cell. 1997b;90:77–86. - PubMed
-
- Baumann P, Benson FE, West SC. Human Rad51 protein promotes ATP-dependent homologous pairing and strand transfer reactions in vitro. Cell. 1996;87:757–766. - PubMed
-
- Benson FE, West SC. Substrate specificity of the Escherichia coli RuvC protein. Resolution of three- and four-stranded recombination intermediates. J Biol Chem. 1994;269:5195–5201. - PubMed (V体育官网)
-
- Camerini-Otero RD, Hsieh P. Homologous recombination proteins in prokaryotes and eukaryotes. Annu Rev Genet. 1995;29:509–552. - PubMed
V体育官网入口 - Publication types
- V体育平台登录 - Actions
MeSH terms
- Actions (VSports最新版本)
- "VSports手机版" Actions
- "VSports手机版" Actions
- VSports手机版 - Actions
- "VSports app下载" Actions
"V体育官网入口" Substances
- "V体育2025版" Actions
- Actions (VSports)
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
