Defects in limb, craniofacial, and thymic development in Jagged2 mutant mice
- PMID: 9531541
- PMCID: PMC316673
- DOI: VSports注册入口 - 10.1101/gad.12.7.1046
Defects in limb, craniofacial, and thymic development in Jagged2 mutant mice
Abstract
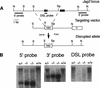
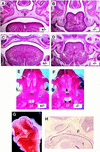
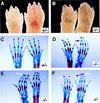
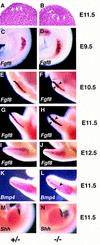
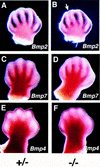
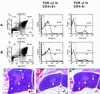
The Notch signaling pathway is a conserved intercellular signaling mechanism that is essential for proper embryonic development in numerous metazoan organisms VSports手机版. We have examined the in vivo role of the Jagged2 (Jag2) gene, which encodes a ligand for the Notch family of transmembrane receptors, by making a targeted mutation that removes a domain of the Jagged2 protein required for receptor interaction. Mice homozygous for this deletion die perinatally because of defects in craniofacial morphogenesis. The mutant homozygotes exhibit cleft palate and fusion of the tongue with the palatal shelves. The mutant mice also exhibit syndactyly (digit fusions) of the fore- and hindlimbs. The apical ectodermal ridge (AER) of the limb buds of the mutant homozygotes is hyperplastic, and we observe an expanded domain of Fgf8 expression in the AER. In the foot plates of the mutant homozygotes, both Bmp2 and Bmp7 expression and apoptotic interdigital cell death are reduced. Mutant homozygotes also display defects in thymic development, exhibiting altered thymic morphology and impaired differentiation of gamma delta lineage T cells. These results demonstrate that Notch signaling mediated by Jag2 plays an essential role during limb, craniofacial, and thymic development in mice. .
Figures (V体育平台登录)


References
-
- Artavanis-Tsakonas S, Matsuno K, Fortini ME. Notch signaling. Science. 1995;268:225–232. - PubMed
-
- Bettenhausen B, Hrabe de Angelis M, Simon D, Guenet J-L, Gossler A. Transient and restricted expression during mouse embryogenesis of Dll1, a murine gene closely related to Drosophila Delta. Development. 1995;121:2407–2418. - PubMed (V体育2025版)
-
- Blaumueller CM, Qi H, Zagouras P, Artavanis-Tsakonas S. Intracellular cleavage of Notch leads to a heterodimeric receptor on the plasma membrane. Cell. 1997;90:281–291. - "V体育平台登录" PubMed
-
- Conlon RA, Reaume AG, Rossant J. Notch1 is required for the coordinate segmentation of somites. Development. 1995;121:1533–1545. - PubMed (VSports app下载)
-
- Crossley PH, Monowada G, MacArthur CA, Martin GR. Roles for FGF8 in the induction, initiation, and maintenance of chick limb development. Cell. 1996;84:127–136. - PubMed
Publication types (V体育官网入口)
- Actions (V体育平台登录)
- "VSports注册入口" Actions
MeSH terms
- "VSports在线直播" Actions
- "V体育平台登录" Actions
- VSports最新版本 - Actions
- "V体育平台登录" Actions
VSports最新版本 - Substances
- Actions (V体育平台登录)
- Actions (V体育安卓版)
Associated data
- "V体育平台登录" Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
