Requirement for NF-kappaB in osteoclast and B-cell development
- PMID: 9407039
- PMCID: PMC316809
- DOI: 10.1101/gad.11.24.3482
Requirement for NF-kappaB in osteoclast and B-cell development
"VSports注册入口" Abstract
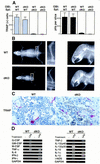
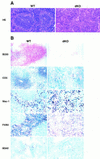
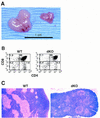
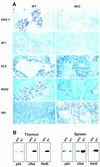
NF-kappaB is a family of related, dimeric transcription factors that are readily activated in cells by signals associated with stress or pathogens. These factors are critical to host defense, as demonstrated previously with mice deficient in individual subunits of NF-kappaB. We have generated mice deficient in both the p50 and p52 subunits of NF-kappaB to reveal critical functions that may be shared by these two highly homologous proteins. We now demonstrate that unlike the respective single knockout mice, the p50/p52 double knockout mice fail to generate mature osteoclasts and B cells, apparently because of defects that track with these lineages in adoptive transfer experiments. Furthermore, these mice present markedly impaired thymic and splenic architectures and impaired macrophage functions. The blocks in osteoclast and B-cell maturation were unexpected VSports手机版. Lack of mature osteoclasts caused severe osteopetrosis, a family of diseases characterized by impaired osteoclastic bone resorption. These findings now establish critical roles for NF-kappaB in development and expand its repertoire of roles in the physiology of differentiated hematopoietic cells. .
Figures




References
-
- Agger R, Witmer-Pack M, Romani N, Stossel H, Swiggard WJ, Metlay JP, Storozynsky E, Freimuth P, Steinman RM. Two populations of splenic dendritic cells detected with M342, a new monoclonal antibody to an intracellular antigen of interdigitating dendritic cells and some B lymphocytes. J Leukocyte Biol. 1992;52:34–42. - PubMed
-
- Anderson G, Moore NC, Owen JJ, Jenkinson EJ. Cellular interactions in thymocyte development. Annu Rev Immunol. 1996;14:73–99. - PubMed
-
- Baeuerle PA, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–179. - V体育安卓版 - PubMed
-
- Baldwin AS. The NF-κB and IκB proteins: New discoveries and insights. Annu Rev Immunol. 1996;14:649–681. - PubMed
-
- Beg AA, Baltimore D. An esssential role for NF-κB in preventing TNF-α-induced cell death. Science. 1996;274:782–784. - PubMed
Publication types (VSports app下载)
VSports手机版 - MeSH terms
- V体育平台登录 - Actions
- Actions (VSports注册入口)
- "V体育官网" Actions
- Actions (VSports)
Substances
- V体育平台登录 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
