Tubulin subunits exist in an activated conformational state generated and maintained by protein cofactors
- PMID: 9265649
- PMCID: "V体育安卓版" PMC2138046
- DOI: 10.1083/jcb.138.4.821
Tubulin subunits exist in an activated conformational state generated and maintained by protein cofactors
Abstract
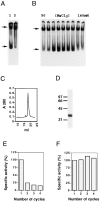
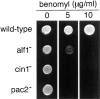
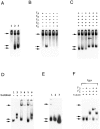
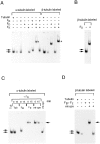
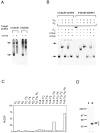
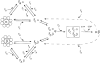
The production of native alpha/beta tubulin heterodimer in vitro depends on the action of cytosolic chaperonin and several protein cofactors. We previously showed that four such cofactors (termed A, C, D, and E) together with native tubulin act on beta-tubulin folding intermediates generated by the chaperonin to produce polymerizable tubulin heterodimers. However, this set of cofactors generates native heterodimers only very inefficiently from alpha-tubulin folding intermediates produced by the same chaperonin. Here we describe the isolation, characterization, and genetic analysis of a novel tubulin folding cofactor (cofactor B) that greatly enhances the efficiency of alpha-tubulin folding in vitro VSports手机版. This enabled an integrated study of alpha- and beta-tubulin folding: we find that the pathways leading to the formation of native alpha- and beta-tubulin converge in that the folding of the alpha subunit requires the participation of cofactor complexes containing the beta subunit and vice versa. We also show that sequestration of native alpha-or beta-tubulins by complex formation with cofactors results in the destabilization and decay of the remaining free subunit. These data demonstrate that tubulin folding cofactors function by placing and/or maintaining alpha-and beta-tubulin polypeptides in an activated conformational state required for the formation of native alpha/beta heterodimers, and imply that each subunit provides information necessary for the proper folding of the other. .
VSports注册入口 - Figures

References
-
- Amberg DC, Botstein D, Beasley EM. Precise gene disruption in Saccharomyces cerevisiaeby double fusion polymerase chain reaction. Yeast. 1996;11:1275–1280. - PubMed
-
- Anfinsen CB. Principles that govern the folding of protein chains. Science (Wash DC) 1973;181:223–230. - PubMed
-
- Archer JE, Vega LR, Solomon F. Rbl2, a yeast protein that binds to β-tubulin and participates in microtubule function in vivo. Cell. 1995;82:425–434. - VSports - PubMed
-
- Baldwin TO, Ziegler MM, Chaffotte AF, Goldberg ME. Contribution of folding steps involving the individual subunits of bacterial luciferase to the assembly of the active heterodimeric enzyme. J Biol Chem. 1993;268:10766–10772. - VSports app下载 - PubMed
-
- Bhattacharyya B, Sackett DL, Wolff J. Tubulin, hybrid dimers, and tubulin S. J Biol Chem. 1985;260:10208–10216. - PubMed
Publication types
- Actions (V体育官网入口)
- "V体育官网" Actions
MeSH terms (V体育ios版)
- Actions (V体育官网入口)
- VSports最新版本 - Actions
- V体育2025版 - Actions
- Actions (V体育安卓版)
- Actions (V体育ios版)
- "VSports最新版本" Actions
- VSports - Actions
- VSports - Actions
- "VSports app下载" Actions
- "V体育官网入口" Actions
- "V体育2025版" Actions
- V体育2025版 - Actions
- V体育2025版 - Actions
- Actions (V体育平台登录)
Substances
- "VSports注册入口" Actions
- Actions (VSports最新版本)
- Actions (V体育2025版)
- Actions (V体育官网入口)
- "V体育ios版" Actions
Associated data
- Actions (VSports app下载)
LinkOut - more resources
VSports最新版本 - Full Text Sources
"VSports手机版" Other Literature Sources
Molecular Biology Databases

