VSports最新版本 - A Randomized Phase II Trial of mFOLFOX6 + Bevacizumab Alone or with AdCEA Vaccine + Avelumab Immunotherapy for Untreated Metastatic Colorectal Cancer
- PMID: 35274710
- PMCID: V体育2025版 - PMC8914498
- DOI: 10.1093/oncolo/oyab046
A Randomized Phase II Trial of mFOLFOX6 + Bevacizumab Alone or with AdCEA Vaccine + Avelumab Immunotherapy for Untreated Metastatic Colorectal Cancer
Abstract
Background: FOLFOX plus bevacizumab is a standard of care (SOC) for first-line treatment of microsatellite-stable metastatic colorectal cancer (MSS mCRC). This study randomized patients to SOC or SOC plus avelumab (anti-PD-L1) plus CEA-targeted vaccine. VSports手机版.
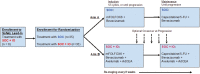
Methods: Patients with untreated MSS mCRC enrolled to a lead-in arm assessing safety of SOC + immuno-oncology agents (IO). Next, patients were randomized to SOC or SOC + IO. The primary endpoint was progression-free survival (PFS). Multiple immune parameters were analyzed V体育安卓版. .
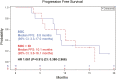
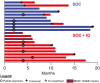
Results: Six patients enrolled to safety lead-in, 10 randomized to SOC, and 10 to SOC + IO. There was no difference in median PFS comparing SOC versus SOC + IO (8. 8 months (95% CI: 3. 3-17. 0 months) versus 10 V体育ios版. 1 months (95% CI: 3. 6-16. 1 months), respectively; hazard ratio 1. 061 [P = . 91; 95% CI: 0. 380-2. 966]). The objective response rate was 50% in both arms. Of patients analyzed, most (8/11) who received SOC + IO developed multifunctional CD4+/CD8+ T-cell responses to cascade antigens MUC1 and/or brachyury, compared to 1/8 who received SOC alone (P = . 020). We detected post-treatment changes in immune parameters that were distinct to the SOC and SOC + IO treatment arms. Accrual closed after an unplanned analysis predicted a low likelihood of meeting the primary endpoint. .
Conclusions: SOC + IO generated multifunctional MUC1- and brachyury-specific CD4+/CD8+ T cells despite concurrent chemotherapy VSports最新版本. Although a tumor-directed immune response is necessary for T-cell-mediated antitumor activity, it was not sufficient to improve PFS. Adding agents that increase the number and function of effector cells may be required for clinical benefit. .
Keywords: Avelumab; FOLFOX; colorectal cancer; combination immunotherapy; immune response; therapeutic vaccine V体育平台登录. .
Published by Oxford University Press 2022 VSports注册入口. This work is written by (a) US Government employee(s) and is in the public domain in the US. .
"V体育安卓版" Figures

References
-
- National Cancer Institute Surveillance, Epiemiology, and End Results Program. National Institutes of Health. Available at https://seer.cancer.gov. Accessed 20 April 2021.
-
- Venook AP, Niedzwiecki D, Lenz HJ, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: a randomized clinical trial. JAMA. 2017;317(23):2392-2401. 10.1001/jama.2017.7105 - DOI - PMC - PubMed
-
- Andre T, Shiu K, Kim T, et al. Pembrolizumab versus chemotherapy for microsatellite instability-high/mismatch repair deficient metastatic colorectal cancer: The phase 3 KEYNOTE-177 Study. J Clin Oncol. 2020;38(suppl 18): Abstract LBA4.
"V体育安卓版" Publication types
- V体育官网入口 - Actions
- "VSports注册入口" Actions
- "VSports在线直播" Actions
MeSH terms
- V体育平台登录 - Actions
- "VSports app下载" Actions
- "V体育ios版" Actions
- V体育ios版 - Actions
- Actions (VSports最新版本)
- Actions (V体育官网入口)
- Actions (VSports app下载)
V体育官网 - Substances
- VSports在线直播 - Actions
- "V体育平台登录" Actions
LinkOut - more resources
VSports注册入口 - Full Text Sources
Medical
Research Materials
Miscellaneous

