Telomerase and Pluripotency Factors Jointly Regulate Stemness in Pancreatic Cancer Stem Cells
- PMID: 34201898
- PMCID: PMC8268125
- DOI: 10.3390/cancers13133145
Telomerase and Pluripotency Factors Jointly Regulate Stemness in Pancreatic Cancer Stem Cells (V体育2025版)
Abstract
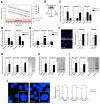
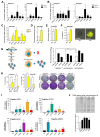
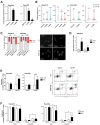
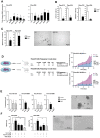
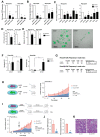
To assess the role of telomerase activity and telomere length in pancreatic CSCs we used different CSC enrichment methods (CD133, ALDH, sphere formation) in primary patient-derived pancreatic cancer cells. We show that CSCs have higher telomerase activity and longer telomeres than bulk tumor cells. Inhibition of telomerase activity, using genetic knockdown or pharmacological inhibitor (BIBR1532), resulted in CSC marker depletion, abrogation of sphere formation in vitro and reduced tumorigenicity in vivo. Furthermore, we identify a positive feedback loop between stemness factors (NANOG, OCT3/4, SOX2, KLF4) and telomerase, which is essential for the self-renewal of CSCs. Disruption of the balance between telomerase activity and stemness factors eliminates CSCs via induction of DNA damage and apoptosis in primary patient-derived pancreatic cancer samples, opening future perspectives to avoid CSC-driven tumor relapse. In the present study, we demonstrate that telomerase regulation is critical for the "stemness" maintenance in pancreatic CSCs and examine the effects of telomerase inhibition as a potential treatment option of pancreatic cancer. This may significantly promote our understanding of PDAC tumor biology and may result in improved treatment for pancreatic cancer patients VSports手机版. .
Keywords: cancer stem cells; pancreatic cancer; self-renewal; stemness; telomerase; telomere length. V体育安卓版.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References (V体育安卓版)
Grants and funding (V体育官网入口)
LinkOut - more resources
V体育2025版 - Full Text Sources
Research Materials

