Phase II Single-arm Study of Durvalumab and Tremelimumab with Concurrent Radiotherapy in Patients with Mismatch Repair-proficient Metastatic Colorectal Cancer
- PMID: 33504552
- PMCID: PMC8499477
- DOI: VSports手机版 - 10.1158/1078-0432.CCR-20-2474
Phase II Single-arm Study of Durvalumab and Tremelimumab with Concurrent Radiotherapy in Patients with Mismatch Repair-proficient Metastatic Colorectal Cancer
Abstract
Purpose: Immune checkpoint inhibition (ICI) alone is not active in mismatch repair-proficient (MMR-P) metastatic colorectal cancer (mCRC), nor does radiotherapy alone result in objective systemic benefit. However, combined radiotherapy plus ICI can induce systemic antitumor immunity in preclinical and clinical models VSports手机版. .
Patients and methods: In this single-center, phase II study, patients with chemotherapy-refractory MMR-P mCRC received durvalumab 1,500 mg plus tremelimumab 75 mg every 4 weeks plus radiotherapy. The primary endpoint was objective response rate (ORR) in nonirradiated lesions V体育安卓版. Treatment and efficacy were correlated with peripheral immune cell profiles. .
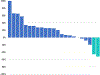
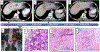
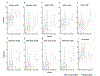
Results: We enrolled 24 patients, and report outcomes after a median follow-up of 21. 8 (range: 15. 9-26. 3) months. The ORR was 8 V体育ios版. 3% (2 patients) [95% confidence interval (CI), 1. 0-27. 0]. The median progression-free survival was 1. 8 (95% CI, 1. 7-1. 9) months, median overall survival was 11. 4 (95% CI, 10. 1-17. 4) months. Twenty five percent of patients (n = 6) had treatment-related grade 3-4 adverse events. We observed increased circulating CD8+ T lymphocyte activation, differentiation, and proliferation in patients with objective response. .
Conclusions: This combination of radiotherapy plus ICI study did not meet the prespecified endpoint criteria to be considered worthwhile for further study. However, rare instances of systemic immune augmentation and regression in nonirradiated lesions were observed (an abscopal response). Combination durvalumab and tremelimumab plus radiotherapy is feasible in MMR-P mCRC with a manageable safety profile. Further studies of novel immunotherapy combinations, and identification of biomarkers predictive of abscopal response are warranted. VSports最新版本.
©2021 American Association for Cancer Research. V体育平台登录.
Figures
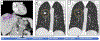
VSports在线直播 - References
-
- Eng C, Kim TW, Bendell J, Argilés G, Tebbutt NC, Di Bartolomeo M, et al. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): a multicentre, open-label, phase 3, randomised, controlled trial. The lancet oncology. 2019;20(6):849–61. - PubMed
-
- Sharma A, Bode B, Studer G, Moch H, Okoniewski M, Knuth A, et al. Radiotherapy of human sarcoma promotes an intratumoral immune effector signature. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19(17):4843–53. - V体育平台登录 - PubMed
Publication types
- "VSports手机版" Actions
- "VSports" Actions
MeSH terms
- Actions (V体育2025版)
- "VSports最新版本" Actions
- Actions (VSports)
- "V体育2025版" Actions
- Actions (VSports app下载)
- VSports - Actions
- "V体育平台登录" Actions
- "VSports" Actions
- Actions (V体育2025版)
- "V体育ios版" Actions
- "VSports在线直播" Actions
- "V体育2025版" Actions
- "VSports最新版本" Actions
- "V体育2025版" Actions
"V体育官网" Substances
- V体育2025版 - Actions
- "V体育官网" Actions
"VSports手机版" Grants and funding
LinkOut - more resources
"VSports注册入口" Full Text Sources
Other Literature Sources
Medical
Research Materials

