Favipiravir: A new and emerging antiviral option in COVID-19 (VSports在线直播)
- PMID: 32895599
- PMCID: "VSports" PMC7467067
- DOI: 10.1016/j.mjafi.2020.08.004
"VSports app下载" Favipiravir: A new and emerging antiviral option in COVID-19
Abstract (V体育2025版)
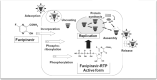
With over 16 million cases reported from across the globe, the SARS-CoV-2, a mere 125 microns in diameter, has left an indelible impact on our world VSports手机版. With the paucity of new drugs to combat this disease, the medical community is in a race to identify repurposed drugs that may be effective against this novel coronavirus. One of the drugs which has recently garnered much attention, especially in India, is an anti-viral drug originally designed for influenza, called favipiravir. In this article, we have tried to provide a comprehensive, evidence-based review of this drug in the context of the present pandemic to elucidate its role in the management of COVID-19. .
Keywords: Antiviral drugs; COVID-19; Favipiravir; SARS-CoV-2 V体育安卓版. .
© 2020 Director General, Armed Forces Medical Services V体育ios版. Published by Elsevier, a division of RELX India Pvt. Ltd. .
Conflict of interest statement
The authors have none to declare.
Figures
"VSports在线直播" References
-
- Search of: favipiravir | Covid19 - list results - clinicaltrials.Gov. 2020. https://clinicaltrials.gov/ct2/results?cond=Covid19&term=favipiravir&cnt... Clinicaltrials.gov. [online] Available at:
-
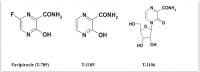
- Furuta Y., Gowen B.B., Takahashi K., Shiraki K., Smee D.F., Barnard D.L. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antivir Res. 2013 Nov;100:446–454. - "V体育安卓版" PMC - PubMed
-
- Toyama Chemicals. Summary of Product Characteristics of Avigan.
-
- Madelain V., Nguyen T.H., Olivo A. Ebola virus infection: review of the pharmacokinetic and pharmacodynamic properties of drugs considered for testing in human efficacy trials. Clin Pharmacokinet. 2016 Aug;55:907–923. doi: 10.1007/s40262-015-0364-1. - "VSports在线直播" DOI - PMC - PubMed
"V体育官网入口" Publication types
LinkOut - more resources
Full Text Sources (VSports最新版本)
Other Literature Sources
Miscellaneous