A Phase II Study of Avelumab Monotherapy in Patients with Mismatch Repair-Deficient/Microsatellite Instability-High or POLE-Mutated Metastatic or Unresectable Colorectal Cancer
- PMID: 32340084
- PMCID: PMC7577804
- DOI: 10.4143/crt.2020.218
A Phase II Study of Avelumab Monotherapy in Patients with Mismatch Repair-Deficient/Microsatellite Instability-High or POLE-Mutated Metastatic or Unresectable Colorectal Cancer
Abstract (V体育安卓版)
Purpose: We evaluated the efficacy and safety of avelumab, an anti-PD-L1 antibody, in patients with metastatic or unresectable colorectal cancer (mCRC) with mismatch repair deficiency (dMMR)/microsatellite instability-high (MSI-H) or POLE mutations. VSports手机版.
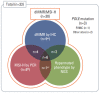
Materials and methods: In this prospective, open-label, multicenter phase II study, 33 patients with mCRC harboring dMMR/MSI-H or POLE mutations after failure of ≥1st-line chemotherapy received avelumab 10 mg/kg every 2 weeks. dMMR/MSI-H was confirmed with immunohistochemical staining (IHC) by loss of expression of MMR proteins or polymerase chain reaction (PCR) for microsatellite sequences. POLE mutation was confirmed by next-generation sequencing (NGS). The primary endpoint was the objective response rate (ORR) by Response Evaluation Criteria in Solid Tumors ver. 1. 1. V体育安卓版.
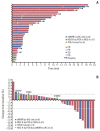
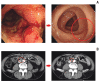
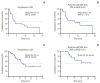
Results: The median age was 60 years, and 78. 8% were male. Thirty patients were dMMR/MSI-H and three had POLE mutations. The ORR was 24. 2%, and all of the responders were dMMR/MSI-H. For 21 patients with MSI-H by PCR or NGS, the ORR was 28. 6%. At a median follow-up duration of 16 V体育ios版. 3 months, median progression-free survival and overall survival were 3. 9 and 13. 2 months in all patients, and 8. 1 months and not reached, respectively, in patients with MSI-H by PCR or NGS. Dose interruption and discontinuation due to treatment-related adverse events occurred in four and two patients, respectively, with no treatment-related deaths. .
Conclusion: Avelumab displayed antitumor activity with manageable toxicity in patients with previously treated mCRC harboring dMMR/MSI-H. Diagnosis of dMMR/MSI-H with PCR or NGS could be complementary to IHC to select patients who would benefit from immunotherapy. VSports最新版本.
Keywords: Avelumab; Colorectal neoplasms; Microsatellite instability; Mismatch repair deficiency; POLE mutation. V体育平台登录.
V体育官网 - Conflict of interest statement
Avelumab was kindly provided by Merck Korea, Seoul, Korea; an affiliate of Merck KGaA, Darmstadt, Germany, as part of an alliance between Merck KGaA and Pfizer VSports注册入口. Merck KGaA, Darmstadt, Germany and Pfizer reviewed the manuscript for medical accuracy only before journal submission.
Figures
"VSports" References
-
- Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–12. - "V体育官网入口" PubMed
-
- Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909–19. - PubMed
Publication types
- "V体育平台登录" Actions
- V体育官网 - Actions
MeSH terms
- "VSports app下载" Actions
- "VSports" Actions
- "V体育官网入口" Actions
- "VSports手机版" Actions
- "V体育平台登录" Actions
- "V体育平台登录" Actions
- "VSports注册入口" Actions
- "V体育安卓版" Actions
- "VSports手机版" Actions
- V体育官网 - Actions
- Actions (V体育官网入口)
- "VSports在线直播" Actions
- VSports在线直播 - Actions
Substances
- Actions (V体育平台登录)
- "V体育官网入口" Actions
- "V体育安卓版" Actions
- VSports注册入口 - Actions
"VSports手机版" Grants and funding
V体育官网 - LinkOut - more resources
Full Text Sources (V体育官网入口)
"V体育ios版" Medical
Research Materials (V体育2025版)

