Flavonoids as Anticancer Agents
- PMID: 32059369
- PMCID: PMC7071196
- DOI: 10.3390/nu12020457
Flavonoids as Anticancer Agents
Abstract
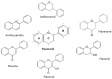
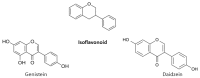
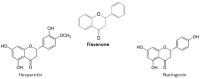
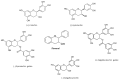
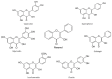
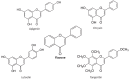
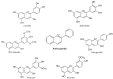
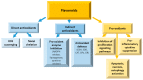
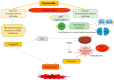
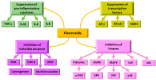
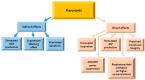
Flavonoids are polyphenolic compounds subdivided into 6 groups: isoflavonoids, flavanones, flavanols, flavonols, flavones and anthocyanidins found in a variety of plants. Fruits, vegetables, plant-derived beverages such as green tea, wine and cocoa-based products are the main dietary sources of flavonoids. Flavonoids have been shown to possess a wide variety of anticancer effects: they modulate reactive oxygen species (ROS)-scavenging enzyme activities, participate in arresting the cell cycle, induce apoptosis, autophagy, and suppress cancer cell proliferation and invasiveness. Flavonoids have dual action regarding ROS homeostasis-they act as antioxidants under normal conditions and are potent pro-oxidants in cancer cells triggering the apoptotic pathways and downregulating pro-inflammatory signaling pathways. This article reviews the biochemical properties and bioavailability of flavonoids, their anticancer activity and its mechanisms of action VSports手机版. .
Keywords: ROS; antioxidants; cancer; flavonoids; mitochondria; pro-oxidants. V体育安卓版.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
"VSports app下载" References
-
- Liu J., Wang X., Yong H., Kan J., Jin C. Recent advances in flavonoid-grafted polysaccharides: Synthesis, structural characterization, bioactivities and potential applications. Int. J. Biol. Macromol. 2018;116:1011–1025. doi: 10.1016/j.ijbiomac.2018.05.149. - VSports在线直播 - DOI - PubMed
-
- Braicu C., Ladomery M.R., Chedea V.S., Irimie A., Berindan-Neagoe I. The relationship between the structure and biological actions of green tea catechins. Food Chem. 2013;141:3282–3289. doi: 10.1016/j.foodchem.2013.05.122. - VSports在线直播 - DOI - PubMed
Publication types
- "V体育官网入口" Actions
MeSH terms
- "VSports最新版本" Actions
- "V体育2025版" Actions
- VSports注册入口 - Actions
- "V体育2025版" Actions
- "V体育2025版" Actions
- "VSports在线直播" Actions
- V体育ios版 - Actions
- V体育安卓版 - Actions
- VSports在线直播 - Actions
- "VSports手机版" Actions
- "V体育官网入口" Actions
- Actions (V体育平台登录)
Substances
- V体育官网入口 - Actions
- "VSports最新版本" Actions
- Actions (V体育ios版)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

