"VSports最新版本" Downregulation of GPSM2 is associated with primary resistance to paclitaxel in breast cancer
- PMID: 32020211
- PMCID: V体育安卓版 - PMC7041173
- DOI: 10.3892/or.2020.7471
Downregulation of GPSM2 is associated with primary resistance to paclitaxel in breast cancer
Abstract
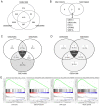
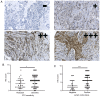
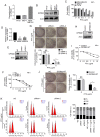
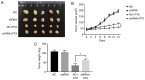
Paclitaxel is one of the most effective chemotherapy drugs for breast cancer worldwide but 20‑30% patients show primary resistance to the drug. Screening and identification of markers that facilitate effective and rapid prediction of sensitivity to paclitaxel is therefore an urgent medical requirement. In the present study, G protein signaling modulator 2 (GPSM2) mRNA levels were significantly associated with taxane sensitivity in experiments based on the Gene Expression Omnibus (GEO) online database. Immunohistochemical analysis consistently revealed a significant association of GPSM2 protein levels with paclitaxel sensitivity in breast cancer patients. Knockdown of GPSM2 reduced the sensitivity of breast cancer cells to paclitaxel via regulation of the cell cycle. Animal experiments further corroborated our in vitro findings. These results suggest that GPSM2 plays an important role in breast cancer resistance, supporting its utility as a potential target for improving drug susceptibility in patients as well as a marker of paclitaxel sensitivity. VSports手机版.
Keywords: breast cancer; GPSM2; paclitaxel; cell cycle V体育安卓版. .
Figures
References
"V体育ios版" MeSH terms
- Actions (V体育平台登录)
- VSports手机版 - Actions
- "V体育2025版" Actions
- "VSports在线直播" Actions
- "VSports" Actions
- "V体育2025版" Actions
- Actions (VSports最新版本)
- "V体育安卓版" Actions
- Actions (V体育官网)
- "V体育官网" Actions
V体育2025版 - Substances
- V体育安卓版 - Actions
- Actions (VSports最新版本)
- "VSports" Actions
- V体育平台登录 - Actions

