The PI3K subunits, P110α and P110β are potential targets for overcoming P-gp and BCRP-mediated MDR in cancer
- PMID: 31952518
- PMCID: PMC6966863
- DOI: 10.1186/s12943-019-1112-1
The PI3K subunits, P110α and P110β are potential targets for overcoming P-gp and BCRP-mediated MDR in cancer
Abstract
Background: PI3K/AKT is a vital signaling pathway in humans VSports手机版. Recently, several PI3K/AKT inhibitors were reported to have the ability to reverse cancer multidrug resistance (MDR); however, specific targets in the PI3K/AKT pathways and the mechanisms associated with MDR have not been found because many of the inhibitors have multiple targets within a large candidate protein pool. AKT activation is one presumed mechanism by which MDR develops during cancer treatment. .
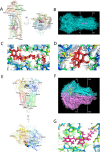
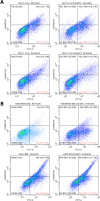
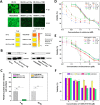
Methods: The effects of inhibiting PI3K 110α and 110β by BAY-1082439 treatment and CRISPR/Cas9 knockout were examined to determine the possible functions of BAY-1082439 and the roles of PI3K 110α and 110β in the reversal of MDR that is mediated by the downregulation of P-gp and BCRP. Inhibition of AKT with GSK-2110183 showed that the downregulation of P-gp and BCRP is independent of generalized AKT inactivation V体育安卓版. Immunofluorescence, immunoprecipitation, MTT, flow cytometry and JC-1 staining analyses were conducted to study the reversal of MDR that is mediated by P-gp and BCRP in cancer cells. An ATPase assay and a structural analysis were also used to analyze the potential mechanisms by which BAY-1082439 specifically targets PI3K 110α and 110β and nonspecifically influences P-gp and BCRP. .
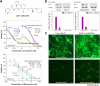
Results: By inhibiting the activation of the PI3K 110α and 110β catalytic subunits through both the administration of BAY-1082439 and the CRISPR/Cas9 deletion of Pik3ca and Pik3cb, the ATP-binding cassette transporters P-gp/ABCB1 and BCRP/ABCG2 were downregulated, thereby reestablishing the drug sensitivity of human epidermoid carcinoma and non-small cell lung cancer (NSCLC) MDR cells. Inhibition of AKT did not reverse the MDR mediated by P-gp or BCRP. The ABC family proteins and AKT may play MDR-enhancing roles independently. V体育ios版.
Conclusions: The reversal of the dual functions of ABC-transporter-mediated and AKT-activation-enhanced MDR through the inhibition or knockout of PI3K 110α or 110β promises to improve current strategies based on combined drug treatments to overcome MDR challenges VSports最新版本. .
Keywords: Breast cancer resistance protein (BCRP/ABCG2/ABCP/MXR); Cancer; Multidrug resistance (MDR); P-glycoprotein (P-gp/ABCB1/MDR1); P110α/PIK3CA; P110β/PIK3CB; PI3K; Reversal of MDR. V体育平台登录.
Conflict of interest statement
The authors declare that they have no competing interests.
V体育ios版 - Figures



References
-
- Cerniglia GJ, Dey S, Gallagher-Colombo SM, Daurio NA, Tuttle S, Busch TM, Lin A, Sun R, Esipova TV, Vinogradov SA, et al. The PI3K/Akt pathway regulates oxygen metabolism via pyruvate dehydrogenase (PDH)-E1alpha phosphorylation. Mol Cancer Ther. 2015;14(8):1928–1938. doi: 10.1158/1535-7163.MCT-14-0888. - DOI - PMC - PubMed
-
- Qian P, He XC, Paulson A, Li Z, Tao F, Perry JM, Guo F, Zhao M, Zhi L, Venkatraman A, et al. The Dlk1-Gtl2 locus preserves LT-HSC function by inhibiting the PI3K-mTOR pathway to restrict mitochondrial metabolism. Cell Stem Cell. 2016;18(2):214–228. doi: 10.1016/j.stem.2015.11.001. - DOI - PMC - PubMed
-
- Liu X. Overstimulation can create health problems due to increases in PI3K/Akt/GSK3 insensitivity and GSK3 activity. Springerplus. 2014;3:356. doi: 10.1186/2193-1801-3-356. - "VSports" DOI - PMC - PubMed
Publication types
V体育平台登录 - MeSH terms
- V体育官网入口 - Actions
- VSports注册入口 - Actions
- Actions (VSports手机版)
- VSports - Actions
- V体育安卓版 - Actions
- "V体育官网" Actions
- Actions (V体育平台登录)
- "V体育ios版" Actions
- Actions (VSports在线直播)
Substances
- "VSports手机版" Actions
- "V体育ios版" Actions
- V体育ios版 - Actions
- V体育安卓版 - Actions
- V体育平台登录 - Actions
- "VSports" Actions
- VSports在线直播 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

