Molecular regulation of Snai2 in development and disease
- PMID: 31792043
- PMCID: "VSports注册入口" PMC12233911
- DOI: 10.1242/jcs.235127
"V体育2025版" Molecular regulation of Snai2 in development and disease
Abstract (VSports最新版本)
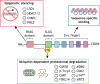
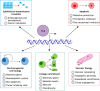
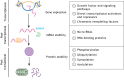
The transcription factor Snai2, encoded by the SNAI2 gene, is an evolutionarily conserved C2H2 zinc finger protein that orchestrates biological processes critical to tissue development and tumorigenesis VSports手机版. Initially characterized as a prototypical epithelial-to-mesenchymal transition (EMT) transcription factor, Snai2 has been shown more recently to participate in a wider variety of biological processes, including tumor metastasis, stem and/or progenitor cell biology, cellular differentiation, vascular remodeling and DNA damage repair. The main role of Snai2 in controlling such processes involves facilitating the epigenetic regulation of transcriptional programs, and, as such, its dysregulation manifests in developmental defects, disruption of tissue homeostasis, and other disease conditions. Here, we discuss our current understanding of the molecular mechanisms regulating Snai2 expression, abundance and activity. In addition, we outline how these mechanisms contribute to disease phenotypes or how they may impact rational therapeutic targeting of Snai2 dysregulation in human disease. .
Keywords: Cancer; Development; Slug. V体育安卓版.
© 2019. Published by The Company of Biologists Ltd V体育ios版. .
Conflict of interest statement
Competing interestsC VSports最新版本. K. is a shareholder of Naveris, Inc. and a member of its scientific board of advisors.
Figures
References
-
- Ambs, S., Prueitt, R. L., Yi, M., Hudson, R. S., Howe, T. M., Petrocca, F., Wallace, T. A., Liu, C.-G., Volinia, S., Calin, G. A., et al. (2008). Genomic profiling of microRNA and messenger RNA reveals deregulated microRNA expression in prostate cancer. Cancer Res. 68, 6162-6170. 10.1158/0008-5472.CAN-08-0144 - DOI - PMC - PubMed
-
- Bai, J.-W., Chen, M.-N., Wei, X.-L., Li, Y.-C., Lin, H.-Y., Chen, M., Li, J.-W., Du, C.-W., Man, K. and Zhang, G.-J. (2017). The zinc-finger transcriptional factor Slug transcriptionally downregulates ERα by recruiting lysine-specific demethylase 1 in human breast cancer. Oncogenesis 6, e330. 10.1038/oncsis.2017.38 - DOI - PMC - PubMed
-
- Barrallo-Gimeno, A. (2005). The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development 132, 3151-3161. 10.1242/dev.01907 - "VSports手机版" DOI - PubMed
"VSports" Publication types
- "VSports app下载" Actions
MeSH terms
- "V体育官网入口" Actions
- "V体育ios版" Actions
- "V体育官网入口" Actions
- VSports最新版本 - Actions
- "VSports" Actions
- VSports app下载 - Actions
V体育ios版 - Substances
- V体育2025版 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources (VSports在线直播)
VSports最新版本 - Other Literature Sources
Research Materials

