Synthesis and Evaluations of "1,4-Triazolyl Combretacoumarins" and Desmethoxy Analogues
- PMID: 31579393
- PMCID: PMC6774347 (V体育官网入口)
- DOI: 10.1002/ejoc.201900569
Synthesis and Evaluations of "1,4-Triazolyl Combretacoumarins" and Desmethoxy Analogues
Abstract
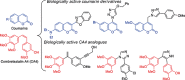
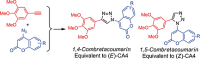
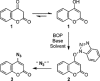
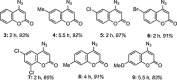
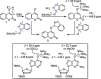
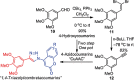
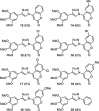
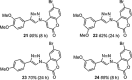
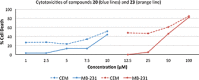
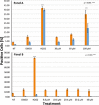
1,4-Triazolyl combretacoumarins have been prepared by linking the trimethoxyarene unit of combretastatin A4 with coumarins, via a 1,2,3-triazole. For this, 4-azidocoumarins were accessed by a sequential two-step, one-pot reaction of 4-hydroxycoumarins with (benzotriazol-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate (BOP), followed by reaction with NaN3. In the reaction with BOP, a coumarin-derived phosphonium ion intermediate seems to form, leading to an O 4-(benzotriazolyl)coumarin derivative. For the CuAAC reaction of azidocoumarins with 5-ethynyl-1,2,3-trimethoxybenzene, catalytic [(MeCN)4Cu]PF6 in CH2Cl2/MeOH with 2,6-lutidine, at 50 °C, was suitable. The 4-azidocoumarins were less reactive as compared to PhN3 and the NBO coefficients of the azido groups were compared by DFT analysis. Compound solubility was a problem in biological assays. On the basis of the biological and solubility data of one 1,4-triazolyl combretacoumarin, four analogues lacking one or two methoxy groups were synthesized. Reactivity differences among the phenylacetylenes were noted and the NBO coefficients of the alkynes were compared by DFT analysis. In antiproliferative assays, 1-phenyl-4-(3,4,5-trimethoxyphenyl)-1H-1,2,3-triazole showed activity in CEM and MDA-MB-231 cell lines, possibly by apoptosis. The desmethoxy 6-bromo-4-(4-(4-methoxyphenyl)-1H-1,2,3-triazol-1-yl)-2H-chromen-2-one also showed cytotoxicity against the two cell lines, but this did not appear to be consistent with apoptosis VSports手机版. The antiviral activity of the compounds was unremarkable. .
Keywords: Coumarin; CuAAC; alkyne; combretastatin; copper; triazole V体育安卓版. .
Figures

References
-
- Advances in Structure and Activity Relationship of Coumarin Derivatives (Ed.: Penta S.), Academic Press, Amsterdam, 2016.
-
- Coumarins: Biology, Applications and Mode of Action (Eds.: O'Kennedy R. and Thornes R. D.), John Wiley & Sons, Chichester, 1997.
-
- Hoult J. R. S. and Payá M., Gen. Pharmacol., 1996, 27, 713–722. - PubMed
-
- Borges F., Roleira F., Milhazes N., Santana L. and Uriarte E., Curr. Med. Chem., 2005, 12, 887–916. - "V体育安卓版" PubMed
-
- Bairagi S. H., Salaskar P. P., Loke S. D., Surve N. N., Tandel D. V. and Dusara M. D., Int. J. Pharm. Res., 2012, 4, 16–19.
Grants and funding
LinkOut - more resources (V体育2025版)
VSports手机版 - Full Text Sources
Miscellaneous
