Effects of the anti-RANKL antibody denosumab on joint structural damage in patients with rheumatoid arthritis treated with conventional synthetic disease-modifying antirheumatic drugs (DESIRABLE study): a randomised, double-blind, placebo-controlled phase 3 trial
- PMID: 31036625
- PMCID: PMC6585575
- DOI: 10.1136/annrheumdis-2018-214827
"V体育官网入口" Effects of the anti-RANKL antibody denosumab on joint structural damage in patients with rheumatoid arthritis treated with conventional synthetic disease-modifying antirheumatic drugs (DESIRABLE study): a randomised, double-blind, placebo-controlled phase 3 trial
Abstract
Objective: To evaluate the efficacy of denosumab in suppressing joint destruction when added to conventional synthetic disease-modifying antirheumatic drug (csDMARD) therapy in patients with rheumatoid arthritis (RA) VSports手机版. .
Methods: This was a multi-centre, randomised, double-blind, parallel-group, placebo-controlled phase 3 study in Japan. Patients with RA aged ≥20 years receiving csDMARDs were randomly assigned (1:1:1) to denosumab 60 mg every 3 months (Q3M), denosumab 60 mg every 6 months (Q6M) or placebo. The change in the modified total Sharp score (mTSS) and effect on bone mineral density (BMD) at 12 months was evaluated. V体育安卓版.
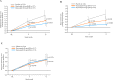
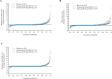
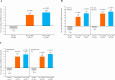
Results: In total, 654 patients received the trial drugs. Denosumab groups showed significantly less progression of joint destruction. The mean changes in the mTSS at 12 months were 1. 49 (95% CI 0. 99 to 1. 99) in the placebo group, 0. 99 (95% CI 0. 49 to 1. 49) in the Q6M group (p=0. 0235) and 0. 72 (95% CI 0. 41 to 1. 03) in the Q3M group (p=0. 0055). The mean changes in bone erosion score were 0. 98 (95% CI 0. 65 to 1. 31) in the placebo group, 0. 51 (95% CI 0. 22 to 0. 80) in the Q6M group (p=0. 0104) and 0. 22 (95% CI 0. 09 to 0. 34) in the Q3M group (p=0. 0001). No significant between-group difference was observed in the joint space narrowing score V体育ios版. The per cent change in lumbar spine (L1-L4) BMD in the placebo, Q6M and Q3M groups were -1. 03%, 3. 99% (p<0. 0001) and 4. 88% (p<0. 0001). No major differences were observed among safety profiles. .
Conclusions: Denosumab inhibits the progression of joint destruction, increases BMD and is well tolerated in patients with RA taking csDMARD. VSports最新版本.
Keywords: denosumab; erosion; joint destruction; rheumatoid arthritis. V体育平台登录.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC VSports注册入口. No commercial re-use. See rights and permissions. Published by BMJ. .
Conflict of interest statement
Competing interests: TT has received research grants from AbbVie, Asahi Kasei, Astellas, AYUMI, Chugai, Daiichi Sankyo, Eisai, Mitsubishi Tanabe, Nippon Kayaku, Novartis, Pfizer and Takeda and has received personal fees from AbbVie, Astellas, Astra Zeneca, Bristol-Myers Squibb, Chugai, Daiichi Sankyo, Eisai, Eli Lilly, GlaxoSmithKline, Janssen, Mitsubishi Tanabe, Nippon Kayaku, Novartis, Pfizer, Sanofi, Taiho, Taisho Toyama, Takeda, Teijin and UCB. YT has received research grants from AbbVie, Astellas, Chugai, Bristol-Myers Squibb, Daiichi Sankyo, Eisai, Kyowa Hakko Kirin, Mitsubishi Tanabe, MSD, Ono, Pfizer and Takeda and has received personal fees from Astellas, Bristol-Myers Squibb, Chugai, Daiichi Sankyo, Eli Lilly, Janssen, Mitsubishi Tanabe, Pfizer, Sanofi, UCB and YL Biologics. SS has received grant/research support from Chugai and Daiichi Sankyo and has received personal fees from Asahi-Kasei Pharma, Astellas, MSD, Chugai, Daiichi Sankyo, Eli Lilly, Mitsubishi-Tanabe, Pfizer, Takeda and Teijin V体育官网入口. HY has received research grants from AbbVie, Astellas, AYUMI, BMS, Chugai, Daiichi Sankyo, Eisai, Kaken, Mitsubishi Tanabe, MSD, Nippon Shinyaku, Ono, Pfizer, Takeda, Teijin, Torii and UCB and has received consulting fees from Astellas, BMS, Chugai, Daiichi Sankyo, Mitsubishi Tanabe, Nippon Kayaku, Pfizer, Takeda, Teijin and YL Biologics. TY has received Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT #17H04377) and has received consulting fees from Daiichi Sankyo. ST has acted as a consultant for AbbVie, Asahi Kasei Pharma, Amgen, Astellas, Daiichi Sankyo, Eli Lilly, MSD, Ono and Teijin Pharma. TN is an employee of Daiichi Sankyo. NO is a shareholder and employee of Daiichi Sankyo. HKG has received consulting fees from Amgen, Agnovos, Bioclinica, Biomarin, Clementia, Daiichi Sanyo, Eli Lilly, Janssen, Medimmune, Merck, Novartis, Pfizer, Regeneron, Servier and Takeda. DvdH has received consulting fees from AbbVie, Amgen, Astellas, AstraZeneca, BMS, Boehringer Ingelheim, Celgene, Daiichi Sankyo, Eli Lilly, Galapagos, Gilead, GlaxoSmithKline, Janssen, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi, Takeda and UCB and is the director of Imaging Rheumatology BV.
VSports注册入口 - Figures

Comment in
-
'DESIRABLE' or not?Ann Rheum Dis. 2021 Aug;80(8):e138. doi: 10.1136/annrheumdis-2019-216236. Epub 2019 Sep 6. Ann Rheum Dis. 2021. PMID: 31492704 No abstract available.
"V体育安卓版" References
-
- Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet 2016;388:2023–38. 10.1016/S0140-6736(16)30173-8 - "V体育2025版" DOI - PubMed
"V体育官网入口" Publication types
- VSports app下载 - Actions
MeSH terms
- Actions (VSports注册入口)
- "V体育2025版" Actions
- V体育安卓版 - Actions
- "V体育官网" Actions
- VSports最新版本 - Actions
- "VSports app下载" Actions
- "V体育2025版" Actions
- VSports - Actions
- Actions (V体育2025版)
- "VSports app下载" Actions
- V体育平台登录 - Actions
- "VSports在线直播" Actions
- VSports app下载 - Actions
- Actions (V体育安卓版)
Substances
- Actions (V体育安卓版)
- VSports注册入口 - Actions

