VSports最新版本 - Icariin Protects Bone Marrow Mesenchymal Stem Cells Against Iron Overload Induced Dysfunction Through Mitochondrial Fusion and Fission, PI3K/AKT/mTOR and MAPK Pathways
- PMID: 30873034
- PMCID: PMC6403125
- DOI: 10.3389/fphar.2019.00163
Icariin Protects Bone Marrow Mesenchymal Stem Cells Against Iron Overload Induced Dysfunction Through Mitochondrial Fusion and Fission, PI3K/AKT/mTOR and MAPK Pathways
Abstract
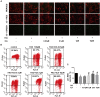
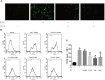
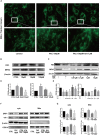
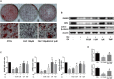
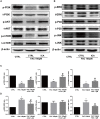
Iron overload has been reported to contribute to bone marrow mesenchymal stem cells (BMSCs) damage, but the precise mechanism still remains elusive. Icariin, a major bioactive monomer belonging to flavonoid glucosides isolated from Herba Epimedii, has been shown to protect cells from oxidative stress induced apoptosis. The aim of this study was to investigate whether icariin protected against iron overload induced dysfunction of BMSCs and its underlying mechanism. In this study, we found that iron overload induced by 100 μM ferric ammonium citrate (FAC) caused apoptosis of BMSCs, promoted cleaved caspase-3 and BAX protein expressions while inhibited Bcl-2 protein expression, which effects were significantly attenuated by icariin treatment. In addition, iron overload induced significant depolarization of mitochondrial membrane potential (MMP), reactive oxygen species (ROS) generation and inhibition of mitochondrial fusion/fission, which effects were also attenuated by icariin treatment. Meanwhile, we found that iron overload induced by 100 μM FAC significantly inhibited mitochondrial fission protein FIS1 and fusion protein MFN2 expressions, inhibited DRP1 and Cytochrome C protein translocation from the cytoplasm to mitochondria. Icariin at concentration of 1 μM was able to promote mitochondrial fission protein FIS1 and fusion protein MFN2 expressions, and increase DRP1 and cytochrome C protein translocation from the cytoplasm to mitochondria. Further, osteogenic differentiation and proliferation of BMSCs was significantly inhibited by iron overload, but icariin treatment rescued both osteogenic differentiation and proliferation of BMSCs. Further studies showed that icariin attenuated iron overload induced inactivation of the PI3K/AKT/mTOR pathway and activation of the ERK1/2 and JNK pathways. In summary, our study indicated that icariin was able to protect against iron overload induced dysfunction of BMSCs. These effects were potentially related to the modulation of mitochondrial fusion and fission, activation of the PI3K/AKT/mTOR pathway and inhibition of ERK1/2 and JNK pathways VSports手机版. .
Keywords: MAPK pathway; PI3K/AKT/mTOR pathway; bone marrow mesenchymal stem cells; icariin; iron overload; mitochondrial fusion and fission V体育安卓版. .
Figures (V体育ios版)


References
-
- Andrews N. C. (2000). Iron metabolism: iron deficiency and iron overload. Annu. Rev. Genomics Hum. Genet. 1 75–98. 10.1146/annurev.genom.1.1.75 - DOI (V体育官网入口) - PubMed
-
- Bresgen N., Eckl P. M. (2015). Oxidative stress and the homeodynamics of iron metabolism. Biomolecules 5 808–847. 10.3390/biom5020808 - DOI (VSports在线直播) - PMC - PubMed
LinkOut - more resources
Full Text Sources (VSports注册入口)
Research Materials
Miscellaneous

