Bovine herpesvirus 4-based vector delivering the full length xCT DNA efficiently protects mice from mammary cancer metastases by targeting cancer stem cells (VSports)
- PMID: 30524888
- PMCID: PMC6279326
- DOI: 10.1080/2162402X.2018.1494108
Bovine herpesvirus 4-based vector delivering the full length xCT DNA efficiently protects mice from mammary cancer metastases by targeting cancer stem cells
Abstract (VSports注册入口)
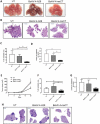
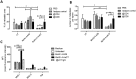
Despite marked advancements in its treatment, breast cancer is still the second leading cause of cancer death in women, due to relapses and distal metastases. Breast cancer stem cells (CSCs), are a cellular reservoir for recurrence, metastatic evolution and disease progression, making the development of novel therapeutics that target CSCs, and thereby inhibit metastases, an urgent need. We have previously demonstrated that the cystine-glutamate antiporter xCT (SLC7A11), a protein that was shown to be overexpressed in mammary CSCs and that plays a key role in the maintenance of their redox balance, self-renewal and resistance to chemotherapy, is a potential target for mammary cancer immunotherapy. This paper reports on the development of an anti-xCT viral vaccine that is based on the bovine herpesvirus 4 (BoHV-4) vector, which we have previously showed to be a safe vaccine that can transduce cells in vivo and confer immunogenicity to tumor antigens VSports手机版. We show that the vaccination of BALB/c mice with BoHV-4 expressing xCT (BoHV-4-mxCT), impaired lung metastases induced by syngeneic mammary CSCs both in preventive and therapeutic settings. Vaccination induced T lymphocyte activation and the production of anti-xCT antibodies that can mediate antibody-dependent cell cytotoxicity (ADCC), and directly impair CSC phenotype, self-renewal and redox balance. Our findings pave the way for the potential future use of BoHV-4-based vector targeting xCT in metastatic breast cancer treatment. .
Keywords: Mammary cancer; bovine herpesvirus 4-based vector; cancer stem cell; immunotherapy; xCT V体育安卓版. .
Figures





References
-
- Colleoni M, Sun Z, Price KN, Karlsson P, Forbes JF, Thurlimann B, Gianni L, Castiglione M, Gelber RD, Coates AS, et al. Annual hazard rates of recurrence for breast cancer during 24 years of follow-up: results from the international breast cancer study group trials I to V. J Clin Oncol. 2016;34:927–935. doi:10.1200/JCO.2015.62.3504. - DOI - PMC - PubMed
-
- Peitzsch C, Tyutyunnykova A, Pantel K, Dubrovska A. Cancer stem cells: the root of tumor recurrence and metastases. Semin Cancer Biol. 2017;44:10–24. doi:10.1016/j.semcancer.2017.02.011. - VSports注册入口 - DOI - PubMed
