"V体育安卓版" Quantitative assessment of cell population diversity in single-cell landscapes
- PMID: 30346945
- PMCID: PMC6211764
- DOI: "V体育平台登录" 10.1371/journal.pbio.2006687
Quantitative assessment of cell population diversity in single-cell landscapes
Abstract
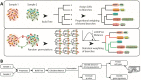
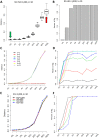
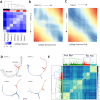
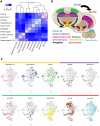
Single-cell RNA sequencing (scRNA-seq) has become a powerful tool for the systematic investigation of cellular diversity. As a number of computational tools have been developed to identify and visualize cell populations within a single scRNA-seq dataset, there is a need for methods to quantitatively and statistically define proportional shifts in cell population structures across datasets, such as expansion or shrinkage or emergence or disappearance of cell populations. Here we present sc-UniFrac, a framework to statistically quantify compositional diversity in cell populations between single-cell transcriptome landscapes. sc-UniFrac enables sensitive and robust quantification in simulated and experimental datasets in terms of both population identity and quantity VSports手机版. We have demonstrated the utility of sc-UniFrac in multiple applications, including assessment of biological and technical replicates, classification of tissue phenotypes and regional specification, identification and definition of altered cell infiltrates in tumorigenesis, and benchmarking batch-correction tools. sc-UniFrac provides a framework for quantifying diversity or alterations in cell populations across conditions and has broad utility for gaining insight into tissue-level perturbations at the single-cell resolution. .
"VSports" Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Gierahn TM, Wadsworth MH, Hughes TK, Bryson BD, Butler A, Satija R, et al. Seq-Well: Portable, low-cost rna sequencing of single cells at high throughput. Nat Methods. 2017;14(4):395–398. 10.1038/nmeth.4179 - "V体育平台登录" DOI - PMC - PubMed
Publication types
- VSports在线直播 - Actions
MeSH terms
- VSports手机版 - Actions
- V体育官网入口 - Actions
- VSports手机版 - Actions
- Actions (V体育安卓版)
- VSports注册入口 - Actions
- VSports手机版 - Actions
- Actions (VSports在线直播)
- Actions (V体育安卓版)
- "V体育安卓版" Actions
- "VSports在线直播" Actions
Grants and funding
- T32 LM012412/LM/NLM NIH HHS/United States
- "VSports" P50 CA095103/CA/NCI NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- R01 DK065949/DK/NIDDK NIH HHS/United States
- V体育ios版 - P50 CA098131/CA/NCI NIH HHS/United States
- U2C CA233291/CA/NCI NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- P50 CA236733/CA/NCI NIH HHS/United States
- F31 GM120940/GM/NIGMS NIH HHS/United States
- R01 DK103831/DK/NIDDK NIH HHS/United States
- R01 DK125696/DK/NIDDK NIH HHS/United States
- U01 CA215798/CA/NCI NIH HHS/United States
- T32 HD007502/HD/NICHD NIH HHS/United States (V体育官网)
- VSports app下载 - R35 CA197570/CA/NCI NIH HHS/United States
- U24 CA163056/CA/NCI NIH HHS/United States
LinkOut - more resources
VSports app下载 - Full Text Sources
Molecular Biology Databases
Research Materials

