Cancer-associated fibroblasts contribute to oral cancer cells proliferation and metastasis via exosome-mediated paracrine miR-34a-5p
- PMID: 30243489
- PMCID: PMC6197737
- DOI: 10.1016/j.ebiom.2018.09.006 (V体育安卓版)
Cancer-associated fibroblasts contribute to oral cancer cells proliferation and metastasis via exosome-mediated paracrine miR-34a-5p
Abstract
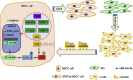
Background: Cancer-associated fibroblasts (CAFs) play an important role in regulating tumor progression by transferring exosomes to neighboring cells VSports手机版. Our aim was to clarify the role of microRNA encapsulated in the exosomes derived from CAFs in oral squamous cell carcinoma (OSCC). .
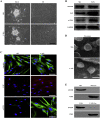
Methods: We examined the microRNA expression profiles of exosomes derived from CAFs and donor-matched normal fibroblasts (NFs) from patients with OSCC. We used confocal microscopy to examine the transportation of exosomal miR-34a-5p between CAFs and OSCC cells V体育安卓版. Next, luciferase reporter and its mutant plasmids were used to confirm direct target gene of miR-34a-5p. Phenotypic assays and in vivo tumor growth experiments were used to investigate the functional significance of exosomal miR-34a-5p. .
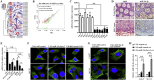
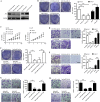
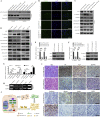
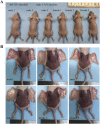
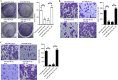
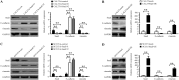
Findings: We found that the expression of miR-34a-5p in CAF-derived exosomes was significantly reduced, and fibroblasts could transfer exosomal miR-34a-5p to OSCC cells. In xenograft experiments, miR-34a-5p overexpression in CAFs could inhibit the tumorigenesis of OSCC cells. We further revealed that miR-34a-5p binds to its direct downstream target AXL to suppress OSCC cell proliferation and metastasis. Stable ectopic expression of AXL in OSCC cells overexpressing miR-34a-5p restored proliferation and motility abolished by the miRNA. The miR-34a-5p/AXL axis promoted OSCC progression via the AKT/GSK-3β/β-catenin signaling pathway, which could induce the epithelial-mesenchymal transition (EMT) to promote cancer cells metastasis. The miR-34a-5p/AXL axis enhanced nuclear translocation of β-catenin and then induced transcriptional upregulation of SNAIL, which in turn activated both MMP-2 and MMP-9. V体育ios版.
Interpretation: The miR-34a-5p/AXL axis confers aggressiveness in oral cancer cells through the AKT/GSK-3β/β-catenin/Snail signaling cascade and might represent a therapeutic target for OSCC. FUND: National Natural Science Foundation of China. VSports最新版本.
Keywords: AXL; CAFs; Exosomes; Oral squamous cell carcinoma; miR-34a-5p V体育平台登录. .
Copyright © 2018 The Authors. Published by Elsevier B. V VSports注册入口. All rights reserved. .
Figures




References
-
- Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309–316. - VSports在线直播 - PubMed
-
- Li Y.Y., Zhou C.X., Gao Y. Moesin regulates the motility of oral cancer cells via MT1-MMP and E-cadherin/p120-catenin adhesion complex. Oral Oncol. 2015;51:935–943. - PubMed
-
- Tlsty T.D., Coussens L.M. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–150. - PubMed
-
- Kalluri R., Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. - PubMed
"VSports注册入口" MeSH terms
- "V体育ios版" Actions
- VSports最新版本 - Actions
- Actions (VSports最新版本)
- VSports注册入口 - Actions
- "V体育平台登录" Actions
- VSports注册入口 - Actions
- VSports在线直播 - Actions
- "V体育安卓版" Actions
- "V体育官网" Actions
- Actions (V体育平台登录)
- Actions (VSports)
- "V体育安卓版" Actions
- "VSports在线直播" Actions
- V体育官网入口 - Actions
- VSports在线直播 - Actions
- "V体育安卓版" Actions
Substances
- "V体育平台登录" Actions
- VSports注册入口 - Actions
- "V体育官网入口" Actions
- V体育平台登录 - Actions
"VSports在线直播" LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

