Novel Selective Estrogen Receptor Ligand Conjugates Incorporating Endoxifen-Combretastatin and Cyclofenil-Combretastatin Hybrid Scaffolds: Synthesis and Biochemical Evaluation
- PMID: 28858267
- PMCID: "V体育官网入口" PMC6151695
- DOI: 10.3390/molecules22091440 (VSports手机版)
Novel Selective Estrogen Receptor Ligand Conjugates Incorporating Endoxifen-Combretastatin and Cyclofenil-Combretastatin Hybrid Scaffolds: Synthesis and Biochemical Evaluation
Abstract
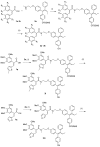
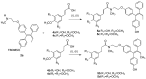
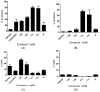
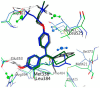
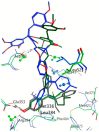
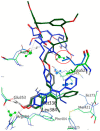
Nuclear receptors such as the estrogen receptors (ERα and ERβ) modulate the effects of the estrogen hormones and are important targets for design of innovative chemotherapeutic agents for diseases such as breast cancer and osteoporosis. Conjugate and bifunctional compounds which incorporate an ER ligand offer a useful method of delivering cytotoxic drugs to tissue sites such as breast cancers which express ERs. A series of novel conjugate molecules incorporating both the ER ligands endoxifen and cyclofenil-endoxifen hybrids covalently linked to the antimitotic and tubulin targeting agent combretastatin A-4 were synthesised and evaluated as ER ligands. A number of these compounds demonstrated pro-apoptotic effects, with potent antiproliferative activity in ER-positive MCF-7 breast cancer cell lines and low cytotoxicity. These conjugates displayed binding affinity towards ERα and ERβ isoforms at nanomolar concentrations e. g. , the cyclofenil-amide compound 13e is a promising lead compound of a clinically relevant ER conjugate with IC50 in MCF-7 cells of 187 nM, and binding affinity to ERα (IC50 = 19 nM) and ERβ (IC50 = 229 nM) while the endoxifen conjugate 16b demonstrates antiproliferative activity in MCF-7 cells (IC50 = 5. 7 nM) and binding affinity to ERα (IC50 = 15 nM) and ERβ (IC50 = 115 nM). The ER binding effects are rationalised in a molecular modelling study in which the disruption of the ER helix-12 in the presence of compounds 11e, 13e and 16b is presented These conjugate compounds have potential application for further development as antineoplastic agents in the treatment of ER positive breast cancers. VSports手机版.
Keywords: apoptosis; combretastatin A-4(CA-4); cyclofenil; endoxifen; estrogen receptor ligands; hormone-dependent breast cancer; selective estrogen receptor modulators; tumour targeting conjugates V体育安卓版. .
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Pasqualini J.R., Chetrite G.S. Recent insight on the control of enzymes involved in estrogen formation and transformation in human breast cancer. J. Steroid Biochem. Mol. Biol. 2005;93:221–236. doi: 10.1016/j.jsbmb.2005.02.007. - "VSports最新版本" DOI - PubMed
-
- Jordan V.C. Antiestrogens and selective estrogen receptor modulators as multifunctional medicines. 1. Receptor interactions. J. Med. Chem. 2003;46:883–908. doi: 10.1021/jm020449y. - "VSports app下载" DOI - PubMed
VSports最新版本 - MeSH terms
- "V体育官网" Actions
- Actions (V体育官网)
- V体育平台登录 - Actions
- VSports app下载 - Actions
- "V体育官网入口" Actions
- Actions (VSports最新版本)
Substances
- Actions (V体育安卓版)
- "V体育平台登录" Actions
- Actions (V体育平台登录)
- "V体育2025版" Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

