Inhibiting the osteocyte-specific protein sclerostin increases bone mass and fracture resistance in multiple myeloma
- PMID: 28515094
- PMCID: PMC5492093
- DOI: 10.1182/blood-2017-03-773341
Inhibiting the osteocyte-specific protein sclerostin increases bone mass and fracture resistance in multiple myeloma
Abstract
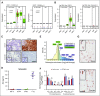
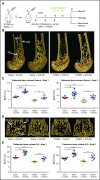
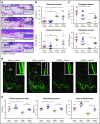
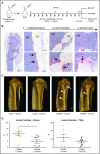
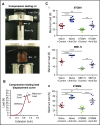
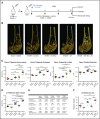
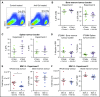
Multiple myeloma (MM) is a plasma cell cancer that develops in the skeleton causing profound bone destruction and fractures. The bone disease is mediated by increased osteoclastic bone resorption and suppressed bone formation. Bisphosphonates used for treatment inhibit bone resorption and prevent bone loss but fail to influence bone formation and do not replace lost bone, so patients continue to fracture. Stimulating bone formation to increase bone mass and fracture resistance is a priority; however, targeting tumor-derived modulators of bone formation has had limited success. Sclerostin is an osteocyte-specific Wnt antagonist that inhibits bone formation. We hypothesized that inhibiting sclerostin would prevent development of bone disease and increase resistance to fracture in MM. Sclerostin was expressed in osteocytes from bones from naive and myeloma-bearing mice. In contrast, sclerostin was not expressed by plasma cells from 630 patients with myeloma or 54 myeloma cell lines. Mice injected with 5TGM1-eGFP, 5T2MM, or MM1. S myeloma cells demonstrated significant bone loss, which was associated with a decrease in fracture resistance in the vertebrae. Treatment with anti-sclerostin antibody increased osteoblast numbers and bone formation rate but did not inhibit bone resorption or reduce tumor burden. Treatment with anti-sclerostin antibody prevented myeloma-induced bone loss, reduced osteolytic bone lesions, and increased fracture resistance. Treatment with anti-sclerostin antibody and zoledronic acid combined increased bone mass and fracture resistance when compared with treatment with zoledronic acid alone. This study defines a therapeutic strategy superior to the current standard of care that will reduce fractures for patients with MM VSports手机版. .
© 2017 by The American Society of Hematology. V体育安卓版.
Figures
VSports在线直播 - References
-
- Becker N. Epidemiology of multiple myeloma. Recent Results Cancer Res. 2011;183:25-35. - PubMed
-
- Röllig C, Knop S, Bornhäuser M. Multiple myeloma. Lancet. 2015;385(9983):2197-2208. - PubMed
-
- Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33. - PubMed
-
- Avet-Loiseau H, Attal M, Moreau P, et al. Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myélome. Blood. 2007;109(8):3489-3495. - V体育ios版 - PubMed
-
- Melton LJ III, Kyle RA, Achenbach SJ, Oberg AL, Rajkumar SV. Fracture risk with multiple myeloma: a population-based study. J Bone Miner Res. 2005;20(3):487-493. - PubMed (VSports)
MeSH terms
- VSports最新版本 - Actions
- "VSports注册入口" Actions
- "V体育ios版" Actions
- "V体育安卓版" Actions
- Actions (V体育安卓版)
- "VSports app下载" Actions
- "VSports手机版" Actions
Substances
- V体育平台登录 - Actions
- "VSports手机版" Actions
- "VSports最新版本" Actions
- VSports - Actions
- VSports - Actions

