Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration
- PMID: 28212525
- PMCID: PMC5312549
- DOI: 10.1016/j.redox.2017.01.021
Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration
Abstract
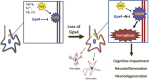
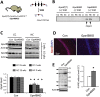
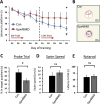
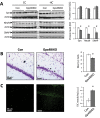
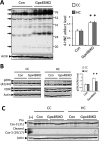
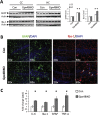
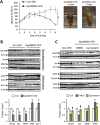
Synaptic loss and neuron death are the underlying cause of neurodegenerative diseases such as Alzheimer's disease (AD); however, the modalities of cell death in those diseases remain unclear. Ferroptosis, a newly identified oxidative cell death mechanism triggered by massive lipid peroxidation, is implicated in the degeneration of neurons populations such as spinal motor neurons and midbrain neurons VSports手机版. Here, we investigated whether neurons in forebrain regions (cerebral cortex and hippocampus) that are severely afflicted in AD patients might be vulnerable to ferroptosis. To this end, we generated Gpx4BIKO mouse, a mouse model with conditional deletion in forebrain neurons of glutathione peroxidase 4 (Gpx4), a key regulator of ferroptosis, and showed that treatment with tamoxifen led to deletion of Gpx4 primarily in forebrain neurons of adult Gpx4BIKO mice. Starting at 12 weeks after tamoxifen treatment, Gpx4BIKO mice exhibited significant deficits in spatial learning and memory function versus Control mice as determined by the Morris water maze task. Further examinations revealed that the cognitively impaired Gpx4BIKO mice exhibited hippocampal neurodegeneration. Notably, markers associated with ferroptosis, such as elevated lipid peroxidation, ERK activation and augmented neuroinflammation, were observed in Gpx4BIKO mice. We also showed that Gpx4BIKO mice fed a diet deficient in vitamin E, a lipid soluble antioxidant with anti-ferroptosis activity, had an expedited rate of hippocampal neurodegeneration and behavior dysfunction, and that treatment with a small-molecule ferroptosis inhibitor ameliorated neurodegeneration in those mice. Taken together, our results indicate that forebrain neurons are susceptible to ferroptosis, suggesting that ferroptosis may be an important neurodegenerative mechanism in diseases such as AD. .
Keywords: Alzheimer's disease; Cognitive impairment; Ferroptosis; Glutathione peroxidase 4; Neurodegeneration; Transgenic mice. V体育安卓版.
Copyright © 2017 V体育ios版. Published by Elsevier B. V. .
"VSports" Figures
References
-
- Cotman C.W., Anderson A.J. A potential role for apoptosis in neurodegeneration and Alzheimer's disease. Mol. Neurobiol. 1995;10(1):19–45. - PubMed
-
- Mattson M.P. Neuronal life-and-death signaling, apoptosis, and neurodegenerative disorders. Antioxid. Redox Signal. 2006;8(11–12):1997–2006. - PubMed
-
- Wang X., Wang W., Li L., Perry G., Lee H.G., Zhu X. Oxidative stress and mitochondrial dysfunction in Alzheimer's disease. Biochim. Biophys. Acta. 2013 - PMC (V体育安卓版) - PubMed
-
- Gordon P.H., Moore D.H., Miller R.G., Florence J.M., Verheijde J.L., Doorish C., Hilton J.F., Spitalny G.M., MacArthur R.B., Mitsumoto H. Efficacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomised trial. Lancet Neurol. 2007;6(12):1045–1053. - PubMed
MeSH terms
- Actions (VSports在线直播)
- "VSports注册入口" Actions
- VSports - Actions
- Actions (VSports注册入口)
- Actions (V体育ios版)
- Actions (V体育ios版)
- V体育官网入口 - Actions
- "VSports在线直播" Actions
- Actions (V体育官网入口)
- V体育2025版 - Actions
- V体育2025版 - Actions
"VSports手机版" Substances
- "VSports" Actions
- V体育官网 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

