"VSports app下载" A Phase I/II Study of the Investigational Drug Alisertib in Combination With Abiraterone and Prednisone for Patients With Metastatic Castration-Resistant Prostate Cancer Progressing on Abiraterone
- PMID: 28178640
- PMCID: PMC5189628
- DOI: "VSports" 10.1634/theoncologist.2016-0297
VSports注册入口 - A Phase I/II Study of the Investigational Drug Alisertib in Combination With Abiraterone and Prednisone for Patients With Metastatic Castration-Resistant Prostate Cancer Progressing on Abiraterone
Abstract
Lessons learned: Patients with metastatic castration-resistant prostate cancer did not tolerate the combination of alisertib with abiraterone and prednisone VSports手机版. There was no clear signal indicating that adding alisertib might be beneficial for those patients progressing on abiraterone. .
Background: We hypothesized that Aurora A kinase (AK) contributes to castrate resistance in prostate cancer (PCa) and that inhibiting AK with alisertib can resensitize PCa cells to androgen receptor (AR) inhibitor abiraterone. V体育安卓版.
Methods: This was a phase I/II trial to determine the safety and efficacy of alisertib when given in combination with abiraterone plus prednisone (AP) V体育ios版. Metastatic castration-resistant prostate cancer (mCRPC) patients were treated with dose escalation (alisertib at 30, 40, and 50 mg orally b. i. d. , days 1-7 every 21 days) per standard 3+3 design. .
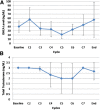
Results: Nine of 43 planned subjects were enrolled. The maximum tolerated dose (MTD) was not reached, and the dose-limiting toxicities (DLTs) included neutropenic fever (1 of 9), neutropenia (1 of 9), fatigue with memory impairment (1 of 9), and diarrhea/mucositis (1 of 9) VSports最新版本. No prostate-specific antigen (PSA) decrease or circulating tumor cell (CTC) changes were observed during the study. Pharmacodynamically, adding alisertib did not affect total testosterone or dehydroepiandrosterone (DHEA) levels. There was some change in neuroendocrine markers after therapy. Mean duration on study was 2. 5 months. The trial was terminated early. .
Conclusion: A tolerable dose of alisertib in combination with AP in mCRPC was not established in this study. There was no clear signal indicating that alisertib might be beneficial for patients with mCRPC progressing on abiraterone. V体育平台登录.
作者总结
经验
• 转移性去势抵抗性前列腺癌患者不能耐受alisertib与阿比特龙和泼尼松的联合方案, VSports注册入口。
• 在接受阿比特龙治疗后发生进展的患者中, 尚无明确迹象表明联合alisertib治疗可带来临床获益, V体育官网入口。
摘要
背景 VSports在线直播, 假设Aurora A激酶(AK)可促成前列腺癌(PCa)发生去势抵抗, 而使用alisertib抑制AK可使PCa细胞对雄激素受体(AR)抑制剂阿比特龙治疗恢复敏感。 。
方法. 本项I/II期临床试验旨在确定alisertib联合阿比特龙和泼尼松(AP)治疗的安全性和有效性。研究为标准3+3设计, 转移性去势抵抗性前列腺癌(mCRPC)患者接受剂量递增治疗: alisertib 30、40和50 mg口服, 每日2次, 第1∼7天, 21天为一周期。
结果. 计划招募43例患者, 研究共纳入9例。本研究未达到最大耐受剂量(MTD), 剂量限制毒性(DLT)包括中性粒细胞减少性发热(1/9例)、中性粒细胞减少(1/9例)、疲乏伴记忆受损(1/9例), 以及腹泻/黏膜炎(1/9例)。研究期间未观察到前列腺特异性抗原(PSA)水平下降及循环肿瘤细胞(CTC)数量改变。从药效学上来说, 联合alisertib没有影响总睾酮或脱氢表雄酮(DHEA)水平。治疗后神经内分泌标记物有所变化。治疗平均时间为2.5个月。研究提早终止。
结论. 本研究未确立alisertib联合AP在mCRPC患者中的耐受剂量。没有明确的征象提示alisertib治疗可为阿比特龙治疗后发生进展的mCRPC患者带来临床获益。The Oncologist 2016;21:1296–1297e
Trial registration: ClinicalTrials.gov NCT01848067.
©AlphaMed Press; the data published online to support this summary is the property of the authors.
Figures
"VSports注册入口" References
-
- Dees EC, Cohen RB, von Mehren M, et al. Phase I study of aurora A kinase inhibitor MLN8237 in advanced solid tumors: Safety, pharmacokinetics, pharmacodynamics, and bioavailability of two oral formulations. Clin Cancer Res. 2012;18:4775–4784. - PubMed
-
- Melichar B, Adenis A, Lockhart AC, et al. Safety and activity of alisertib, an investigational aurora kinase A inhibitor, in patients with breast cancer, small-cell lung cancer, non-small-cell lung cancer, head and neck squamous-cell carcinoma, and gastro-oesophageal adenocarcinoma: A five-arm phase 2 study. Lancet Oncol. 2015;16:395–405. - PubMed
Publication types
MeSH terms
- "V体育2025版" Actions
- "VSports app下载" Actions
- VSports在线直播 - Actions
- V体育官网 - Actions
- "V体育2025版" Actions
- Actions (VSports最新版本)
- Actions (V体育官网)
Substances
- Actions (V体育官网)
- "V体育2025版" Actions
Associated data
- "VSports app下载" Actions
LinkOut - more resources
"V体育2025版" Full Text Sources
Other Literature Sources
Medical
"V体育官网入口" Research Materials
Miscellaneous