Cardiac myofibroblast engulfment of dead cells facilitates recovery after myocardial infarction
- PMID: 27918308
- PMCID: PMC5199696
- DOI: 10.1172/JCI83822
VSports手机版 - Cardiac myofibroblast engulfment of dead cells facilitates recovery after myocardial infarction
Abstract
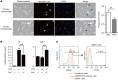
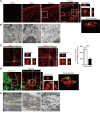
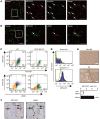
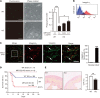
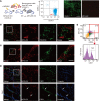
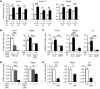
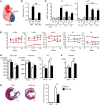
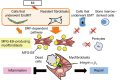
Myocardial infarction (MI) results in the generation of dead cells in the infarcted area VSports手机版. These cells are swiftly removed by phagocytes to minimize inflammation and limit expansion of the damaged area. However, the types of cells and molecules responsible for the engulfment of dead cells in the infarcted area remain largely unknown. In this study, we demonstrated that cardiac myofibroblasts, which execute tissue fibrosis by producing extracellular matrix proteins, efficiently engulf dead cells. Furthermore, we identified a population of cardiac myofibroblasts that appears in the heart after MI in humans and mice. We found that these cardiac myofibroblasts secrete milk fat globule-epidermal growth factor 8 (MFG-E8), which promotes apoptotic engulfment, and determined that serum response factor is important for MFG-E8 production in myofibroblasts. Following MFG-E8-mediated engulfment of apoptotic cells, myofibroblasts acquired antiinflammatory properties. MFG-E8 deficiency in mice led to the accumulation of unengulfed dead cells after MI, resulting in exacerbated inflammatory responses and a substantial decrease in survival. Moreover, MFG-E8 administration into infarcted hearts restored cardiac function and morphology. MFG-E8-producing myofibroblasts mainly originated from resident cardiac fibroblasts and cells that underwent endothelial-mesenchymal transition in the heart. Together, our results reveal previously unrecognized roles of myofibroblasts in regulating apoptotic engulfment and a fundamental importance of these cells in recovery from MI. .
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures (V体育安卓版)


References
-
- Roger VL, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. - DOI (V体育官网入口) - PMC - PubMed
-
- Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357(11):1121–1135. doi: 10.1056/NEJMra071667. - DOI (V体育ios版) - PubMed
-
- Whelan RS, Kaplinskiy V, Kitsis RN. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol. 2010;72:19–44. doi: 10.1146/annurev.physiol.010908.163111. - "VSports手机版" DOI - PubMed
-
- Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res. 2012;110(1):159–173. doi: 10.1161/CIRCRESAHA.111.243162. - DOI (V体育2025版) - PMC - PubMed
Publication types
- V体育官网入口 - Actions
MeSH terms
- Actions (VSports最新版本)
- Actions (V体育平台登录)
- Actions (V体育ios版)
- V体育官网入口 - Actions
- "VSports" Actions
- V体育平台登录 - Actions
- Actions (V体育ios版)
- "V体育ios版" Actions
- Actions (VSports最新版本)
- Actions (V体育2025版)
Substances (V体育平台登录)
LinkOut - more resources
"VSports手机版" Full Text Sources
Other Literature Sources
Medical
"V体育官网入口" Molecular Biology Databases
Research Materials
Miscellaneous

