VSports注册入口 - Homeostasis of the gut barrier and potential biomarkers
- PMID: 27908847
- PMCID: PMC5440615 (VSports手机版)
- DOI: 10.1152/ajpgi.00048.2015
Homeostasis of the gut barrier and potential biomarkers
Abstract
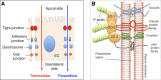
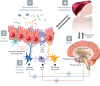
The gut barrier plays a crucial role by spatially compartmentalizing bacteria to the lumen through the production of secreted mucus and is fortified by the production of secretory IgA (sIgA) and antimicrobial peptides and proteins. With the exception of sIgA, expression of these protective barrier factors is largely controlled by innate immune recognition of microbial molecular ligands. Several specialized adaptations and checkpoints are operating in the mucosa to scale the immune response according to the threat and prevent overreaction to the trillions of symbionts inhabiting the human intestine. A healthy microbiota plays a key role influencing epithelial barrier functions through the production of short-chain fatty acids (SCFAs) and interactions with innate pattern recognition receptors in the mucosa, driving the steady-state expression of mucus and antimicrobial factors. However, perturbation of gut barrier homeostasis can lead to increased inflammatory signaling, increased epithelial permeability, and dysbiosis of the microbiota, which are recognized to play a role in the pathophysiology of a variety of gastrointestinal disorders. Additionally, gut-brain signaling may be affected by prolonged mucosal immune activation, leading to increased afferent sensory signaling and abdominal symptoms. In turn, neuronal mechanisms can affect the intestinal barrier partly by activation of the hypothalamus-pituitary-adrenal axis and both mast cell-dependent and mast cell-independent mechanisms. The modulation of gut barrier function through nutritional interventions, including strategies to manipulate the microbiota, is considered a relevant target for novel therapeutic and preventive treatments against a range of diseases VSports手机版. Several biomarkers have been used to measure gut permeability and loss of barrier integrity in intestinal diseases, but there remains a need to explore their use in assessing the effect of nutritional factors on gut barrier function. Future studies should aim to establish normal ranges of available biomarkers and their predictive value for gut health in human cohorts. .
Keywords: antimicrobial peptides; epithelial permeability; gut barrier; microbiota V体育安卓版. .
Copyright © 2017 the American Physiological Society V体育ios版. .
Figures (V体育安卓版)

References (VSports最新版本)
-
- Adams RB, Planchon SM, Roche JK. IFN-gamma modulation of epithelial barrier function. Time course, reversibility, and site of cytokine binding. J Immunol 150: 2356–2363, 1993. - PubMed
-
- Adriaanse MP, Tack GJ, Passos VL, Damoiseaux JG, Schreurs MW, van Wijck K, Riedl RG, Masclee AA, Buurman WA, Mulder CJ, Vreugdenhil AC. Serum I-FABP as marker for enterocyte damage in coeliac disease and its relation to villous atrophy and circulating autoantibodies. Aliment Pharmacol Ther 37: 482–490, 2013. doi:10.1111/apt.12194. - DOI - PubMed
-
- Agus A, Denizot J, Thévenot J, Martinez-Medina M, Massier S, Sauvanet P, Bernalier-Donadille A, Denis S, Hofman P, Bonnet R, Billard E, Barnich N. Western diet induces a shift in microbiota composition enhancing susceptibility to adherent-invasive E. coli infection and intestinal inflammation. Sci Rep 6: 19032, 2016. doi:10.1038/srep19032. - DOI - PMC - PubMed
Publication types
MeSH terms
- VSports注册入口 - Actions
- "V体育ios版" Actions
- Actions (V体育ios版)
- VSports手机版 - Actions
- Actions (VSports注册入口)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

