Multiparametric profiling of non-small-cell lung cancers reveals distinct immunophenotypes
- PMID: 27699239
- PMCID: V体育2025版 - PMC5033841
- DOI: 10.1172/jci.insight.89014
V体育平台登录 - Multiparametric profiling of non-small-cell lung cancers reveals distinct immunophenotypes
Abstract
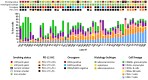
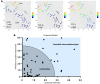
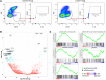
BACKGROUND. Immune checkpoint blockade improves survival in a subset of patients with non-small-cell lung cancer (NSCLC), but robust biomarkers that predict response to PD-1 pathway inhibitors are lacking. Furthermore, our understanding of the diversity of the NSCLC tumor immune microenvironment remains limited. METHODS. We performed comprehensive flow cytometric immunoprofiling on both tumor and immune cells from 51 NSCLCs and integrated this analysis with clinical and histopathologic characteristics, next-generation sequencing, mRNA expression, and PD-L1 immunohistochemistry (IHC). RESULTS. Cytometric profiling identified an immunologically "hot" cluster with abundant CD8+ T cells expressing high levels of PD-1 and TIM-3 and an immunologically "cold" cluster with lower relative abundance of CD8+ T cells and expression of inhibitory markers. The "hot" cluster was highly enriched for expression of genes associated with T cell trafficking and cytotoxic function and high PD-L1 expression by IHC. There was no correlation between immunophenotype and KRAS or EGFR mutation, or patient smoking history, but we did observe an enrichment of squamous subtype and tumors with higher mutation burden in the "hot" cluster VSports手机版. Additionally, approximately 20% of cases had high B cell infiltrates with a subset producing IL-10. CONCLUSIONS. Our results support the use of immune-based metrics to study response and resistance to immunotherapy in lung cancer. FUNDING. The Robert A. and Renée E. Belfer Family Foundation, Expect Miracles Foundation, Starr Cancer Consortium, Stand Up to Cancer Foundation, Conquer Cancer Foundation, International Association for the Study of Lung Cancer, National Cancer Institute (R01 CA205150), and the Damon Runyon Cancer Research Foundation. .
Figures





References
-
- Garon EB, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. - PubMed
-
- EER Stat Fact Sheets: Lung and Bronchus Cancer. National Cancer Institute. VSports app下载 - http://seer.cancer.gov/statfacts/html/lungb.html Accessed August 30, 2016.
-
- Rizvi NA, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16(3):257–265. - VSports手机版 - PMC - PubMed
Publication types
MeSH terms
- Actions (VSports在线直播)
- Actions (V体育官网)
- VSports手机版 - Actions
- Actions (VSports在线直播)
- "V体育2025版" Actions
- VSports - Actions
Substances
- Actions (V体育平台登录)
Grants and funding
LinkOut - more resources
"VSports手机版" Full Text Sources
Other Literature Sources
Medical
V体育ios版 - Molecular Biology Databases
Research Materials
Miscellaneous

