MiR675-5p Acts on HIF-1α to Sustain Hypoxic Responses: A New Therapeutic Strategy for Glioma
- PMID: 27279905
- PMCID: PMC4893639
- DOI: 10.7150/thno.14700
MiR675-5p Acts on HIF-1α to Sustain Hypoxic Responses: A New Therapeutic Strategy for Glioma
"VSports最新版本" Abstract
Hypoxia is a common feature in solid tumours. In glioma, it is considered the major driving force for tumour angiogenesis and correlates with enhanced resistance to conventional therapies, increased invasiveness and a poor prognosis for patients. Here we describe, for the first time, that miR675-5p, embedded in hypoxia-induced long non-coding RNA H19, plays a mandatory role in establishing a hypoxic response and in promoting hypoxia-mediated angiogenesis. We demonstrated, in vitro and in vivo, that miR675-5p over expression in normoxia is sufficient to induce a hypoxic moreover, miR675-5p depletion in low oxygen conditions, drastically abolishes hypoxic responses including angiogenesis VSports手机版. In addition, our data indicate an interaction of miR675-5p, HIF-1α mRNA and the RNA Binding Protein HuR in hypoxia-induced responses. We suggest the modulation of miR675-5p as a new therapeutic option to promote or abolish hypoxia induced angiogenesis. .
Keywords: Angiogenesis; HuR; VHL V体育安卓版. ; glioma; hypoxia; miRNA675; optical imaging. .
Conflict of interest statement
Competing Interests: The authors declare no competing interest.
Figures


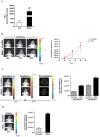
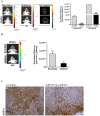
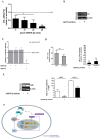
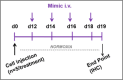

References
-
- Vaupel P, Harrison L. Tumor hypoxia: causative factors, compensatory mechanisms, and cellular response. Oncologist. 2004;9(Suppl 5):4–9. - "V体育ios版" PubMed
-
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. - "VSports手机版" PubMed
-
- Mongiardi MP. Angiogenesis and hypoxia in glioblastoma: a focus on cancer stem cells. CNS Neurol Disord Drug Targets. 2012;11:878–83. - PubMed
-
- Jensen RL. Hypoxia in the tumorigenesis of gliomas and as a potential target for therapeutic measures. Neurosurg Focus. 2006;20:E24. - PubMed
-
- Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D. et al. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830–5. - PubMed
"VSports手机版" Publication types
- "V体育ios版" Actions
MeSH terms
- "V体育平台登录" Actions
- "V体育官网入口" Actions
- "VSports最新版本" Actions
- Actions (VSports)
- Actions (V体育官网入口)
V体育平台登录 - Substances
- Actions (VSports最新版本)
LinkOut - more resources
Full Text Sources
Other Literature Sources (VSports在线直播)
Medical
Miscellaneous

