"VSports app下载" Mechanical signals regulate and activate SNAIL1 protein to control the fibrogenic response of cancer-associated fibroblasts
- PMID: 27076520
- PMCID: "VSports在线直播" PMC4878991
- DOI: 10.1242/jcs.180539
V体育平台登录 - Mechanical signals regulate and activate SNAIL1 protein to control the fibrogenic response of cancer-associated fibroblasts
Erratum in
-
Correction: Mechanical signals regulate and activate SNAIL1 protein to control the fibrogenic response of cancer-associated fibroblasts (doi:10.1242/jcs.180539). (V体育官网)J Cell Sci. 2019 May 2;132(9):jcs232348. doi: 10.1242/jcs.232348. J Cell Sci. 2019. PMID: 31048546 Free PMC article. No abstract available.
Abstract
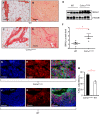
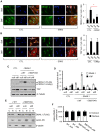
Increased deposition of collagen in extracellular matrix (ECM) leads to increased tissue stiffness and occurs in breast tumors. When present, this increases tumor invasion and metastasis. Precisely how this deposition is regulated and maintained in tumors is unclear VSports手机版. Much has been learnt about mechanical signal transduction in cells, but transcriptional responses and the pathophysiological consequences are just becoming appreciated. Here, we show that the SNAIL1 (also known as SNAI1) protein level increases and accumulates in nuclei of breast tumor cells and cancer-associated fibroblasts (CAFs) following exposure to stiff ECM in culture and in vivo SNAIL1 is required for the fibrogenic response of CAFs when exposed to a stiff matrix. ECM stiffness induces ROCK activity, which stabilizes SNAIL1 protein indirectly by increasing intracellular tension, integrin clustering and integrin signaling to ERK2 (also known as MAPK1). Increased ERK2 activity leads to nuclear accumulation of SNAIL1, and, thus, avoidance of cytosolic proteasome degradation. SNAIL1 also influences the level and activity of YAP1 in CAFs exposed to a stiff matrix. This work describes a mechanism whereby increased tumor fibrosis can perpetuate activation of CAFs to sustain tumor fibrosis and promote tumor metastasis through regulation of SNAIL1 protein level and activity. .
Keywords: Extracellular matrix; Fibrosis; Mechanotransduction; SNAIL1. V体育安卓版.
© 2016. Published by The Company of Biologists Ltd. V体育ios版.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures






References
-
- Aragona M., Panciera T., Manfrin A., Giulitti S., Michielin F., Elvassore N., Dupont S. and Piccolo S. (2013). A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 154, 1047-1059. 10.1016/j.cell.2013.07.042 - DOI (VSports最新版本) - PubMed
-
- Batlle R., Alba-Castellón L., Loubat-Casanovas J., Armenteros E., Francí C., Stanisavljevic J., Banderas R., Martin-Caballero J., Bonilla F., Baulida J. et al. (2013). Snail1 controls TGF-beta responsiveness and differentiation of mesenchymal stem cells. Oncogene 32, 3381-3389. 10.1038/onc.2012.342 - DOI - PMC - PubMed
-
- Bhowmick N. A., Neilson E. G. and Moses H. L. (2004). Stromal fibroblasts in cancer initiation and progression. Nature 432, 332-337. 10.1038/nature03096 - "V体育ios版" DOI - PMC - PubMed
-
- Calvo F., Ege N., Grande-Garcia A., Hooper S., Jenkins R. P., Chaudhry S. I., Harrington K., Williamson P., Moeendarbary E., Charras G. et al. (2013). Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol. 15, 637-646. 10.1038/ncb2756 - "VSports手机版" DOI - PMC - PubMed
MeSH terms (VSports在线直播)
- Actions (V体育官网)
- V体育安卓版 - Actions
- "V体育平台登录" Actions
- Actions (V体育平台登录)
- VSports注册入口 - Actions
Substances
- "VSports注册入口" Actions
- V体育ios版 - Actions
- Actions (V体育官网)
- Actions (V体育2025版)
- Actions (VSports app下载)
- VSports注册入口 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
V体育2025版 - Medical
Research Materials (VSports app下载)
Miscellaneous

