The EGF Receptor Promotes the Malignant Potential of Glioma by Regulating Amino Acid Transport System xc(-) (VSports app下载)
- PMID: 26980765
- PMCID: PMC4873328 (VSports最新版本)
- DOI: "VSports在线直播" 10.1158/0008-5472.CAN-15-2121
The EGF Receptor Promotes the Malignant Potential of Glioma by Regulating Amino Acid Transport System xc(-) (V体育官网入口)
Abstract
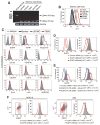
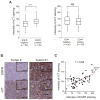
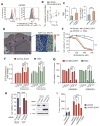
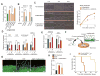
Extracellular free amino acids contribute to the interaction between a tumor and its microenvironment through effects on cellular metabolism and malignant behavior. System xc(-) is composed of xCT and CD98hc subunits and functions as a plasma membrane antiporter for the uptake of extracellular cystine in exchange for intracellular glutamate VSports手机版. Here, we show that the EGFR interacts with xCT and thereby promotes its cell surface expression and function in human glioma cells. EGFR-expressing glioma cells manifested both enhanced antioxidant capacity as a result of increased cystine uptake, as well as increased glutamate, which promotes matrix invasion. Imaging mass spectrometry also revealed that brain tumors formed in mice by human glioma cells stably overexpressing EGFR contained higher levels of reduced glutathione compared with those formed by parental cells. Targeted inhibition of xCT suppressed the EGFR-dependent enhancement of antioxidant capacity in glioma cells, as well as tumor growth and invasiveness. Our findings establish a new functional role for EGFR in promoting the malignant potential of glioma cells through interaction with xCT at the cell surface. Cancer Res; 76(10); 2954-63. ©2016 AACR. .
©2016 American Association for Cancer Research V体育安卓版. .
Conflict of interest statement
Figures

"V体育安卓版" References
-
- Al-Zhoughbi W, Huang J, Paramasivan GS, Till H, Pichler M, Guertl-Lackner B, et al. Tumor macroenvironment and metabolism. Semin Oncol. 2014;41:281–95. - VSports注册入口 - PMC - PubMed
-
- Cantor JR, Sabatini DM. Cancer cell metabolism: one hallmark, many faces. Cancer Discov. 2012;2:881–98. - VSports最新版本 - PMC - PubMed
-
- Zhang W, Trachootham D, Liu J, Chen G, Pelicano H, Garcia-Prieto C, et al. Stromal control of cystine metabolism promotes cancer cell survival in chronic lymphocytic leukaemia. Nat Cell Biol. 2012;14:276–86. - VSports app下载 - PMC - PubMed
-
- Ganapathy V, Thangaraju M, Prasad PD. Nutrient transporters in cancer: relevance to Warburg hypothesis and beyond. Pharmacol Ther. 2009;121:29–40. - PubMed
-
- Sato H, Shiiya A, Kimata M, Maebara K, Tamba M, Sakakura Y, et al. Redox imbalance in cystine/glutamate transporter-deficient mice. J Biol Chem. 2005;280:37423–9. - PubMed
MeSH terms
- "V体育2025版" Actions
- VSports在线直播 - Actions
- V体育ios版 - Actions
- Actions (VSports)
- Actions (VSports在线直播)
- Actions (VSports注册入口)
- "V体育安卓版" Actions
- VSports手机版 - Actions
- "V体育官网入口" Actions
- "V体育2025版" Actions
- V体育平台登录 - Actions
- "VSports最新版本" Actions
Substances
- Actions (V体育安卓版)
- Actions (VSports手机版)
- V体育官网 - Actions
- VSports app下载 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials (V体育ios版)
Miscellaneous (V体育官网入口)

