Rapid evolutionary turnover underlies conserved lncRNA-genome interactions
- PMID: 26773003
- PMCID: PMC4719309
- DOI: V体育官网 - 10.1101/gad.272187.115
"VSports注册入口" Rapid evolutionary turnover underlies conserved lncRNA-genome interactions
Abstract
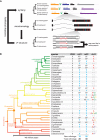
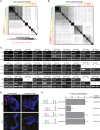
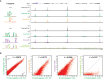
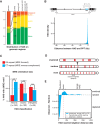
Many long noncoding RNAs (lncRNAs) can regulate chromatin states, but the evolutionary origin and dynamics driving lncRNA-genome interactions are unclear. We adapted an integrative strategy that identifies lncRNA orthologs in different species despite limited sequence similarity, which is applicable to mammalian and insect lncRNAs VSports手机版. Analysis of the roX lncRNAs, which are essential for dosage compensation of the single X chromosome in Drosophila males, revealed 47 new roX orthologs in diverse Drosophilid species across ∼40 million years of evolution. Genetic rescue by roX orthologs and engineered synthetic lncRNAs showed that altering the number of focal, repetitive RNA structures determines roX ortholog function. Genomic occupancy maps of roX RNAs in four species revealed conserved targeting of X chromosome neighborhoods but rapid turnover of individual binding sites. Many new roX-binding sites evolved from DNA encoding a pre-existing RNA splicing signal, effectively linking dosage compensation to transcribed genes. Thus, dynamic change in lncRNAs and their genomic targets underlies conserved and essential lncRNA-genome interactions. .
Keywords: ChIRP; Drosophila; RNA structure; dosage compensation; lncRNAs; roX V体育安卓版. .
© 2016 Quinn et al V体育ios版. ; Published by Cold Spring Harbor Laboratory Press. .
Figures


References
-
- Alekseyenko AA, Peng S, Larschan E, Gorchakov AA, Lee OK, Kharchenko P, McGrath SD, Wang CI, Mardis ER, Park PJ, et al. 2008. A sequence motif within chromatin entry sites directs MSL establishment on the Drosophila X chromosome. Cell 134: 599–609. - "VSports" PMC - PubMed
-
- Alekseyenko AA, Ellison CE, Gorchakov AA, Zhou Q, Kaiser VB, Toda N, Walton Z, Peng S, Park PJ, Bachtrog D, et al. 2013. Conservation and de novo acquisition of dosage compensation on newly evolved sex chromosomes in Drosophila. Genes Dev 27: 853–858. - PMC (V体育官网入口) - PubMed
-
- Amrein H, Axel R. 1997. Genes expressed in neurons of adult male Drosophila. Cell 88: 459–469. - PubMed
VSports注册入口 - Publication types
MeSH terms
- Actions (V体育官网)
- "V体育平台登录" Actions
- "V体育安卓版" Actions
- Actions (VSports app下载)
- "VSports最新版本" Actions
- "V体育官网" Actions
- VSports - Actions
Substances
- VSports在线直播 - Actions
VSports在线直播 - Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
"VSports app下载" Molecular Biology Databases
