Fibrocyte-like cells mediate acquired resistance to anti-angiogenic therapy with bevacizumab
- PMID: 26635184
- PMCID: "V体育ios版" PMC4686833
- DOI: 10.1038/ncomms9792
Fibrocyte-like cells mediate acquired resistance to anti-angiogenic therapy with bevacizumab
Abstract
Bevacizumab exerts anti-angiogenic effects in cancer patients by inhibiting vascular endothelial growth factor (VEGF). However, its use is still limited due to the development of resistance to the treatment. Such resistance can be regulated by various factors, although the underlying mechanisms remain incompletely understood. Here we show that bone marrow-derived fibrocyte-like cells, defined as alpha-1 type I collagen-positive and CXCR4-positive cells, contribute to the acquired resistance to bevacizumab. In mouse models of malignant pleural mesothelioma and lung cancer, fibrocyte-like cells mediate the resistance to bevacizumab as the main producer of fibroblast growth factor 2. In clinical specimens of lung cancer, the number of fibrocyte-like cells is significantly increased in bevacizumab-treated tumours, and correlates with the number of treatment cycles, as well as CD31-positive vessels. Our results identify fibrocyte-like cells as a promising cell biomarker and a potential therapeutic target to overcome resistance to anti-VEGF therapy. VSports手机版.
Conflict of interest statement (V体育安卓版)
Y V体育安卓版. N. has received research support from Chugai and Taiho Pharmaceuticals. The remaining authors declare no competing financial interests.
Figures

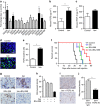
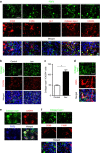
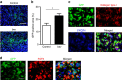
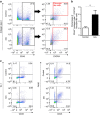
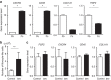
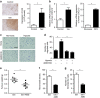
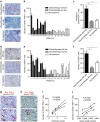
References
-
- Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat. Rev. Drug Discov. 6, 273–286 (2007). - PubMed
-
- Ferrara N., Gerber H. P. & LeCouter J. The biology of VEGF and its receptors. Nat. Med 9, 669–676 (2003). - PubMed
-
- Ferrara N. & Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr. Rev. 18, 4–25 (1997). - PubMed
-
- Sandler A. et al.. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N. Engl. J. Med. 355, 2542–2550 (2006). - PubMed
Publication types
"VSports注册入口" MeSH terms
- V体育官网入口 - Actions
- "VSports手机版" Actions
- Actions (VSports最新版本)
- "VSports注册入口" Actions
- "VSports在线直播" Actions
- "VSports app下载" Actions
Substances
- Actions (VSports在线直播)
- VSports在线直播 - Actions
- Actions (V体育安卓版)
Associated data
- Actions
LinkOut - more resources
"VSports手机版" Full Text Sources
Other Literature Sources
Medical
V体育2025版 - Molecular Biology Databases

