Factors Influencing the Central Nervous System Distribution of a Novel Phosphoinositide 3-Kinase/Mammalian Target of Rapamycin Inhibitor GSK2126458: Implications for Overcoming Resistance with Combination Therapy for Melanoma Brain Metastases
- PMID: 26604245
- PMCID: "VSports app下载" PMC4727156
- DOI: 10.1124/jpet.115.229393
Factors Influencing the Central Nervous System Distribution of a Novel Phosphoinositide 3-Kinase/Mammalian Target of Rapamycin Inhibitor GSK2126458: Implications for Overcoming Resistance with Combination Therapy for Melanoma Brain Metastases (V体育安卓版)
Abstract
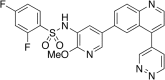
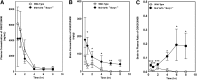
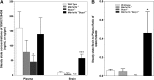
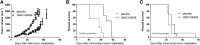
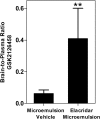
Small molecule inhibitors targeting the mitogen-activated protein kinase pathway (Braf/mitogen-activated protein kinase kinase/extracellular signal-regulated kinase) have had success in extending survival for patients with metastatic melanoma. Unfortunately, resistance may occur via cross-activation of alternate signaling pathways. One approach to overcome resistance is to simultaneously target the phosphoinositide 3-kinase/mammalian target of rapamycin signaling pathway VSports手机版. Recent reports have shown that GSK2126458 [2,4-difluoro-N-(2-methoxy-5-(4-(pyridazin-4-yl)quinolin-6-yl)pyridin-3-yl) benzenesulfonamide], a dual phosphoinositide 3-kinase/mammalian target of rapamycin inhibitor, can overcome acquired resistance to Braf and mitogen-activated protein kinase kinase inhibitors in vitro. These resistance mechanisms may be especially important in melanoma brain metastases because of limited drug delivery across the blood-brain barrier. The purpose of this study was to investigate factors that influence the brain distribution of GSK2126458 and to examine the efficacy of GSK2126458 in a novel patient-derived melanoma xenograft (PDX) model. Both in vitro and in vivo studies indicate that GSK2126458 is a substrate for P-glycoprotein (P-gp) and breast cancer resistance protein (Bcrp), two dominant active efflux transporters in the blood-brain barrier. The steady-state brain distribution of GSK2126458 was 8-fold higher in the P-gp/Bcrp knockout mice compared with the wild type. We also observed that when simultaneously infused to steady state, GSK212658, dabrafenib, and trametinib, a rational combination to overcome mitogen-activated protein kinase inhibitor resistance, all had limited brain distribution. Coadministration of elacridar, a P-gp/Bcrp inhibitor, increased the brain distribution of GSK2126458 by approximately 7-fold in wild-type mice. In the PDX model, GSK2126458 showed efficacy in flank tumors but was ineffective in intracranial melanoma. These results show that P-gp and Bcrp are involved in limiting the brain distribution of GSK2126458 and provide a rationale for the lack of efficacy of GSK2126458 in the orthotopic PDX model. .
Copyright © 2016 by The American Society for Pharmacology and Experimental Therapeutics. V体育安卓版.
Figures


"VSports最新版本" References
-
- Bollag G, Tsai J, Zhang J, Zhang C, Ibrahim P, Nolop K, Hirth P. (2012) Vemurafenib: the first drug approved for BRAF-mutant cancer. Nat Rev Drug Discov 11:873–886. - PubMed
Publication types
- VSports注册入口 - Actions
MeSH terms
- VSports手机版 - Actions
- "V体育ios版" Actions
- VSports最新版本 - Actions
- V体育官网 - Actions
- V体育安卓版 - Actions
- VSports在线直播 - Actions
- V体育平台登录 - Actions
- "VSports手机版" Actions
- V体育2025版 - Actions
- "V体育2025版" Actions
- "VSports在线直播" Actions
- "VSports最新版本" Actions
- Actions (VSports手机版)
Substances (V体育安卓版)
- Actions (V体育安卓版)
- "V体育官网" Actions
Grants and funding
"V体育官网" LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

