Resveratrol inhibits epithelial-mesenchymal transition of retinal pigment epithelium and development of proliferative vitreoretinopathy
- PMID: 26552368
- PMCID: PMC4639835
- DOI: 10.1038/srep16386
Resveratrol inhibits epithelial-mesenchymal transition of retinal pigment epithelium and development of proliferative vitreoretinopathy
Abstract
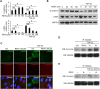
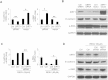
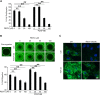
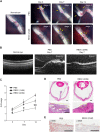
Proliferative vitreoretinopathy (PVR) is a serious complication of retinal detachment and ocular trauma, and its recurrence may lead to irreversible vision loss. Epithelial to mesenchymal transition (EMT) of retinal pigment epithelial (RPE) cells is a critical step in the pathogenesis of PVR, which is characterized by fibrotic membrane formation and traction retinal detachment. In this study, we investigated the potential impact of resveratrol (RESV) on EMT and the fibrotic process in cultured RPE cells and further examined the preventive effect of RESV on PVR development using a rabbit model of PVR. We found that RESV induces mesenchymal to epithelial transition (MET) and inhibits transforming growth factor-β2(TGF-β2)-induced EMT of RPE cells by deacetylating SMAD4. The effect of RESV on MET was dependent on sirtuin1 activation. RESV suppressed proliferation, migration and fibronectin synthesis induced by platelet-derived growth factor-BB or TGF-β2. In vivo, RESV inhibited the progression of experimental PVR in rabbit eyes. Histological findings showed that RESV reduced fibrotic membrane formation and decreased α-SMA expression in the epiretinal membranes. These results suggest the potential use of RESV as a therapeutic agent to prevent the development of PVR by targeting EMT of RPE. VSports手机版.
Figures
References
-
- Ryan S. J. The pathophysiology of proliferative vitreoretinopathy in its management. Am J Ophthalmol 100, 188–193 (1985). - PubMed
-
- Constable I. J. & Nagpal M. In Retina 5th edn, Vol. 3 (eds Ryan S. J. et al.) Ch. 107, p. 1806–1825 (Elsevier, 2013).
-
- Tosi G. M., Marigliani D., Romeo N. & Toti P. Disease pathways in proliferative vitreoretinopathy: an ongoing challenge. J Cell Physiol 229, 1577–1583 (2014). - PubMed
-
- Hiscott P., Sheridan C., Magee R. M. & Grierson I. Matrix and the retinal pigment epithelium in proliferative retinal disease. Prog Retin Eye Res 18, 167–190 (1999). - PubMed
-
- Strauss O. The retinal pigment epithelium in visual function. Physiol. Rev. 85, 845–881 (2005). - "VSports注册入口" PubMed
Publication types
- "VSports app下载" Actions
- VSports app下载 - Actions
"V体育官网入口" MeSH terms
- "VSports" Actions
- Actions (V体育官网)
- VSports最新版本 - Actions
- "VSports手机版" Actions
- VSports在线直播 - Actions
- VSports app下载 - Actions
- "V体育安卓版" Actions
- Actions (VSports最新版本)
- V体育平台登录 - Actions
- V体育ios版 - Actions
- Actions (VSports app下载)
- "VSports最新版本" Actions
- "VSports在线直播" Actions
- Actions (V体育平台登录)
- "V体育安卓版" Actions
V体育ios版 - Substances
- V体育官网 - Actions
- V体育官网入口 - Actions
- V体育官网 - Actions
Grants and funding (VSports最新版本)
"V体育2025版" LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
"VSports手机版" Miscellaneous

