V体育官网入口 - p53-regulated autophagy is controlled by glycolysis and determines cell fate
- PMID: 26337205
- PMCID: PMC4695109
- DOI: 10.18632/oncotarget.5218
p53-regulated autophagy is controlled by glycolysis and determines cell fate
"V体育安卓版" Abstract
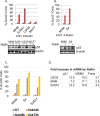
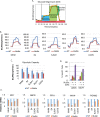
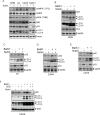
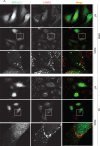
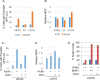
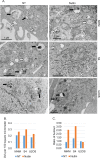
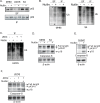
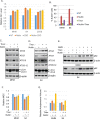
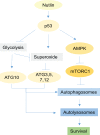
The tumor suppressor p53 regulates downstream targets that determine cell fate. Canonical p53 functions include inducing apoptosis, growth arrest, and senescence VSports手机版. Non-canonical p53 functions include its ability to promote or inhibit autophagy and its ability to regulate metabolism. The extent to which autophagy and/or metabolic regulation determines cell fate by p53 is unclear. To address this, we compared cells resistant or sensitive to apoptosis by the p53 activator Nutlin-3a. In resistant cells, glycolysis was maintained upon Nutlin-3a treatment, and activated p53 promoted prosurvival autophagy. In contrast, in apoptosis sensitive cells activated p53 increased superoxide levels and inhibited glycolysis through repression of glycolytic pathway genes. Glycolysis inhibition and increased superoxide inhibited autophagy by repressing ATG genes essential for autophagic vesicle maturation. Inhibiting glycolysis increased superoxide and blocked autophagy in apoptosis-resistant cells, causing p62-dependent caspase-8 activation. Finally, treatment with 2-DG or the autophagy inhibitors chloroquine or bafilomycin A1 sensitized resistant cells to Nutlin-3a-induced apoptosis. Together, these findings reveal novel links between glycolysis and autophagy that determine apoptosis-sensitivity in response to p53. Specifically, the findings indicate 1) that glycolysis plays an essential role in autophagy by limiting superoxide levels and maintaining expression of ATG genes required for autophagic vesicle maturation, 2) that p53 can promote or inhibit autophagy depending on the status of glycolysis, and 3) that inhibiting protective autophagy can expand the breadth of cells susceptible to Nutlin-3a induced apoptosis. .
Keywords: Nutlin-3a; autophagy; glycolysis; p53. V体育安卓版.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Schuler M, Green DR. Mechanisms of p53-dependent apoptosis. Biochemical Society transactions. 2001;29:684–688. - V体育官网入口 - PubMed
-
- Yu J, Zhang L. The transcriptional targets of p53 in apoptosis control. Biochemical and biophysical research communications. 2005;331:851–858. - VSports手机版 - PubMed
-
- Gartel AL, Serfas MS, Tyner AL. p21--negative regulator of the cell cycle. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine. 1996;213:138–149. - PubMed
-
- Riley T, Sontag E, Chen P, Levine A. Transcriptional control of human p53-regulated genes. Nature reviews Molecular cell biology. 2008;9:402–412. - PubMed
Publication types
MeSH terms (VSports手机版)
- V体育官网 - Actions
- "V体育2025版" Actions
- Actions (VSports注册入口)
- V体育官网 - Actions
- Actions (V体育平台登录)
- "V体育2025版" Actions
- "V体育平台登录" Actions
- V体育ios版 - Actions
- "V体育安卓版" Actions
- "V体育ios版" Actions
Substances
- V体育安卓版 - Actions
- "VSports最新版本" Actions
- Actions (VSports手机版)
- "VSports注册入口" Actions
- "V体育平台登录" Actions
Grants and funding
LinkOut - more resources
Full Text Sources
"VSports注册入口" Other Literature Sources
Research Materials
"V体育ios版" Miscellaneous

