Cutting Edge: Dual Function of PPARγ in CD11c+ Cells Ensures Immune Tolerance in the Airways
- PMID: 26062999
- PMCID: PMC4490989
- DOI: 10.4049/jimmunol.1500474
Cutting Edge: Dual Function of PPARγ in CD11c+ Cells Ensures Immune Tolerance in the Airways
Abstract
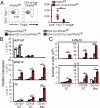
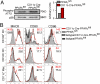
The respiratory tract maintains immune homeostasis despite constant provocation by environmental Ags. Failure to induce tolerogenic responses to allergens incites allergic inflammation. Despite the understanding that APCs have a crucial role in maintaining immune tolerance, the underlying mechanisms are poorly understood. Using mice with a conditional deletion of peroxisome proliferator-activated receptor γ (PPARγ) in CD11c(+) cells, we show that PPARγ performs two critical functions in CD11c(+) cells to induce tolerance, thereby preserving immune homeostasis. First, PPARγ was crucial for the induction of retinaldehyde dehydrogenase (aldh1a2) selectively in CD103(+) dendritic cells, which we recently showed promotes Foxp3 expression in naive CD4(+) T cells. Second, in all CD11c(+) cells, PPARγ was required to suppress expression of the Th17-skewing cytokines IL-6 and IL-23p19. Also, lack of PPARγ in CD11c(+) cells induced p38 MAPK activity, which was recently linked to Th17 development. Thus, PPARγ favors immune tolerance by promoting regulatory T cell generation and blocking Th17 differentiation. VSports手机版.
Copyright © 2015 by The American Association of Immunologists, Inc. V体育安卓版.
Figures

References
-
- Lambrecht BN, Hammad H. Lung dendritic cells in respiratory viral infection and asthma: from protection to immunopathology. Annu. Rev. Immunol. 2012;30:243–270. - "VSports注册入口" PubMed
-
- Steinman RM. Decisions about dendritic cells: past, present, and future. Annu. Rev. Immunol. 2012;30:1–22. - PubMed
-
- Wahli W, Michalik L. PPARs at the crossroads of lipid signaling and inflammation. Trends Endocrinol Metab. 2012;23:351–363. - "V体育安卓版" PubMed
-
- Belvisi MG, Hele DJ, Birrell MA. Peroxisome proliferator-activated receptor gamma agonists as therapy for chronic airway inflammation. Eur J Pharmacol. 2006;533:101–109. - PubMed
Publication types
MeSH terms
- V体育安卓版 - Actions
- Actions (VSports在线直播)
- "V体育2025版" Actions
- Actions (VSports最新版本)
- "V体育ios版" Actions
- "VSports在线直播" Actions
- "VSports手机版" Actions
- "VSports最新版本" Actions
- "VSports手机版" Actions
- Actions (V体育官网)
- V体育官网入口 - Actions
- V体育安卓版 - Actions
- VSports注册入口 - Actions
- "V体育官网入口" Actions
- Actions (VSports最新版本)
- "VSports在线直播" Actions
- V体育安卓版 - Actions
- V体育官网入口 - Actions
Substances (VSports在线直播)
- "VSports最新版本" Actions
- VSports注册入口 - Actions
- VSports app下载 - Actions
- V体育官网 - Actions
- "V体育ios版" Actions
- "VSports手机版" Actions
- "VSports在线直播" Actions
- Actions (VSports)
- Actions (V体育官网入口)
Grants and funding
LinkOut - more resources
Full Text Sources
"V体育官网" Other Literature Sources
Medical
Research Materials
"V体育安卓版" Miscellaneous

