The human NAD metabolome: Functions, metabolism and compartmentalization (VSports)
- PMID: 25837229
- PMCID: PMC4673589 (VSports app下载)
- DOI: "VSports最新版本" 10.3109/10409238.2015.1028612
VSports最新版本 - The human NAD metabolome: Functions, metabolism and compartmentalization
Abstract
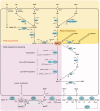
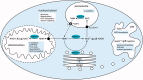
The metabolism of NAD has emerged as a key regulator of cellular and organismal homeostasis. Being a major component of both bioenergetic and signaling pathways, the molecule is ideally suited to regulate metabolism and major cellular events. In humans, NAD is synthesized from vitamin B3 precursors, most prominently from nicotinamide, which is the degradation product of all NAD-dependent signaling reactions. The scope of NAD-mediated regulatory processes is wide including enzyme regulation, control of gene expression and health span, DNA repair, cell cycle regulation and calcium signaling. In these processes, nicotinamide is cleaved from NAD(+) and the remaining ADP-ribosyl moiety used to modify proteins (deacetylation by sirtuins or ADP-ribosylation) or to generate calcium-mobilizing agents such as cyclic ADP-ribose. This review will also emphasize the role of the intermediates in the NAD metabolome, their intra- and extra-cellular conversions and potential contributions to subcellular compartmentalization of NAD pools. VSports手机版.
Keywords: ADP-ribosylation; NAD biosynthesis; calcium signaling; extracellular NAD degradation; protein deacetylation; subcellular NAD pools. V体育安卓版.
Figures
References
-
- Adriouch S, Hubert S, Pechberty S, et al. NAD + released during inflammation participates in T cell homeostasis by inducing ART2-mediated death of naive T cells in vivo. J Immunol. 2007;179:186–94. - PubMed
-
- Adriouch S, Bannas P, Schwarz N, et al. ADP-ribosylation at R125 gates the P2X7 ion channel by presenting a covalent ligand to its nucleotide binding site. FASEB J. 2008;22:861–9. - PubMed
-
- Agrimi G, Russo A, Scarcia P, et al. The human gene SLC25A17 encodes a peroxisomal transporter of coenzyme A, FAD and NAD+ Biochem J. 2012;443:241–7. - PubMed (VSports手机版)
-
- Aksoy S, Szumlanski CL, Weinshilboum RM. Human liver nicotinamide N-methyltransferase. cDNA cloning, expression, and biochemical characterization. J Biol Chem. 1994;269:14835–40. - PubMed (V体育平台登录)
Publication types (V体育官网入口)
"V体育安卓版" MeSH terms
- "V体育平台登录" Actions
- VSports - Actions
- Actions (V体育安卓版)
- V体育ios版 - Actions
- V体育官网 - Actions
- "V体育ios版" Actions
- Actions (VSports在线直播)
VSports注册入口 - Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
