"V体育安卓版" Functionally compromised CHD7 alleles in patients with isolated GnRH deficiency
- PMID: 25472840
- PMCID: PMC4273325
- DOI: "V体育平台登录" 10.1073/pnas.1417438111
Functionally compromised CHD7 alleles in patients with isolated GnRH deficiency
V体育2025版 - Abstract
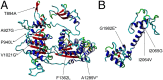
Inactivating mutations in chromodomain helicase DNA binding protein 7 (CHD7) cause CHARGE syndrome, a severe multiorgan system disorder of which Isolated gonadotropin-releasing hormone (GnRH) deficiency (IGD) is a minor feature. Recent reports have described predominantly missense CHD7 alleles in IGD patients, but it is unclear if these alleles are relevant to causality or overall genetic burden of Kallmann syndrome (KS) and normosmic form of IGD. To address this question, we sequenced CHD7 in 783 well-phenotyped IGD patients lacking full CHARGE features; we identified nonsynonymous rare sequence variants in 5. 2% of the IGD cohort (73% missense and 27% splice variants) VSports手机版. Functional analyses in zebrafish using a surrogate otolith assay of a representative set of these CHD7 alleles showed that rare sequence variants observed in controls showed no altered function. In contrast, 75% of the IGD-associated alleles were deleterious and resulted in both KS and normosmic IGD. In two families, pathogenic mutations in CHD7 coexisted with mutations in other known IGD genes. Taken together, our data suggest that rare deleterious CHD7 alleles contribute to the mutational burden of patients with both KS and normosmic forms of IGD in the absence of full CHARGE syndrome. These findings (i) implicate a unique role or preferential sensitivity for CHD7 in the ontogeny of GnRH neurons, (ii) reiterate the emerging genetic complexity of this family of IGD disorders, and (iii) demonstrate how the coordinated use of well-phenotyped cohorts, families, and functional studies can inform genetic architecture and provide insights into the developmental biology of cellular systems. .
Keywords: CHARGE syndrome; CHD7; Kallmann syndrome; idiopathic hypogonadotropic hypogondism; missense mutations. V体育安卓版.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Vissers LE, et al. Mutations in a new member of the chromodomain gene family cause CHARGE syndrome. Nat Genet. 2004;36(9):955–957. - PubMed
-
- Sanlaville D, Verloes A. CHARGE syndrome: An update. Eur J Hum Genet. 2007;15(4):389–399. - PubMed
-
- Bergman JE, et al. CHD7 mutations and CHARGE syndrome: The clinical implications of an expanding phenotype. J Med Genet. 2011;48(5):334–342. - "VSports app下载" PubMed
Publication types
MeSH terms
- VSports - Actions
- Actions (VSports在线直播)
- Actions (V体育ios版)
- Actions (V体育2025版)
- "VSports在线直播" Actions
- V体育官网入口 - Actions
- Actions (V体育平台登录)
- "V体育2025版" Actions
- VSports注册入口 - Actions
- VSports注册入口 - Actions
- Actions (VSports app下载)
- V体育安卓版 - Actions
Substances (VSports注册入口)
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

