Mechanisms controlling the smooth muscle cell death in progeria via down-regulation of poly(ADP-ribose) polymerase 1
- PMID: 24843141
- PMCID: "V体育2025版" PMC4050581
- DOI: 10.1073/pnas.1320843111
"V体育平台登录" Mechanisms controlling the smooth muscle cell death in progeria via down-regulation of poly(ADP-ribose) polymerase 1
Abstract
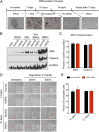
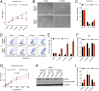
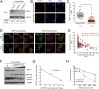
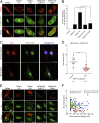
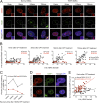
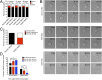
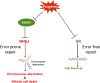
Hutchinson-Gilford progeria syndrome (HGPS) is a severe human premature aging disorder caused by a lamin A mutant named progerin. Death occurs at a mean age of 13 y from cardiovascular problems. Previous studies revealed loss of vascular smooth muscle cells (SMCs) in the media of large arteries in a patient with HGPS and two mouse models, suggesting a causal connection between the SMC loss and cardiovascular malfunction. However, the mechanisms of how progerin leads to massive SMC loss are unknown. In this study, using SMCs differentiated from HGPS induced pluripotent stem cells, we show that HGPS SMCs exhibit a profound proliferative defect, which is primarily caused by caspase-independent cell death. Importantly, progerin accumulation stimulates a powerful suppression of PARP1 and consequently triggers an activation of the error-prone nonhomologous end joining response. As a result, most HGPS SMCs exhibit prolonged mitosis and die of mitotic catastrophe. This study demonstrates a critical role of PARP1 in mediating SMC loss in patients with HGPS and elucidates a molecular pathway underlying the progressive SMC loss in progeria VSports手机版. .
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443(7113):787–795. - PubMed (VSports)
-
- Vakifahmetoglu H, Olsson M, Zhivotovsky B. Death through a tragedy: Mitotic catastrophe. Cell Death Differ. 2008;15(7):1153–1162. - PubMed
Publication types
- Actions (VSports app下载)
MeSH terms (VSports在线直播)
- "VSports最新版本" Actions
- Actions (V体育官网入口)
- "V体育官网" Actions
- Actions (VSports注册入口)
- VSports注册入口 - Actions
- V体育ios版 - Actions
- "VSports注册入口" Actions
- "V体育安卓版" Actions
- Actions (V体育官网)
- "VSports注册入口" Actions
- Actions (V体育官网入口)
Substances (VSports注册入口)
- V体育官网 - Actions
- "V体育官网入口" Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous (V体育安卓版)

