"V体育2025版" A peptide that inhibits function of Myristoylated Alanine-Rich C Kinase Substrate (MARCKS) reduces lung cancer metastasis
- PMID: 23955080
- PMCID: PMC4631387
- DOI: V体育2025版 - 10.1038/onc.2013.336
A peptide that inhibits function of Myristoylated Alanine-Rich C Kinase Substrate (MARCKS) reduces lung cancer metastasis
Abstract
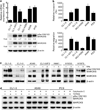
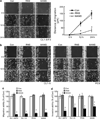
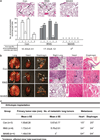
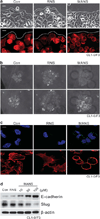
Myristoylated Alanine-Rich C Kinase Substrate (MARCKS), a substrate of protein kinase C, is a key regulatory molecule controlling mucus granule secretion by airway epithelial cells as well as directed migration of leukocytes, stem cells and fibroblasts VSports手机版. Phosphorylation of MARKCS may be involved in these responses. However, the functionality of MARCKS and its related phosphorylation in lung cancer malignancy have not been characterized. This study demonstrated elevated levels of MARCKS and phospho-MARCKS in highly invasive lung cancer cell lines and lung cancer specimens from non-small-cell lung cancer patients. siRNA knockdown of MARCKS expression in these highly invasive lung cancer cell lines reduced cell migration and suppressed PI3K (phosphatidylinositol 3'-kinase)/Akt phosphorylation and Slug level. Interestingly, treatment with a peptide identical to the MARCKS N-terminus sequence (the MANS peptide) impaired cell migration in vitro and also the metastatic potential of invasive lung cancer cells in vivo. Mechanistically, MANS peptide treatment resulted in a coordination of increase of E-cadherin expression, suppression of MARCKS phosphorylation and AKT/Slug signalling pathway but not the expression of total MARCKS. These results indicate a crucial role for MARCKS, specifically its phosphorylated form, in potentiating lung cancer cell migration/metastasis and suggest a potential use of MARCKS-related peptides in the treatment of lung cancer metastasis. .
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Teicher BA. Protein kinase C as a therapeutic target. Clin Cancer Res. 2006;12:5336–5345. - PubMed
-
- Roffey J, Rosse C, Linch M, Hibbert A, McDonald NQ, Parker PJ. Protein kinase C intervention: the state of play. Curr Opin Cell Biol. 2009;21:268–279. - "VSports" PubMed
-
- Harlan DM, Graff JM, Stumpo DJ, Eddy RL, Jr, Shows TB, Boyle JM, et al. The human myristoylated alanine-rich C kinase substrate (MARCKS) gene (MACS). Analysis of its gene product, promoter, and chromosomal localization. J Biol Chem. 1991;266:14399–14405. - PubMed
-
- Thelen M, Rosen A, Nairn AC, Aderem A. Regulation by phosphorylation of reversible association of a myristoylated protein kinase C substrate with the plasma membrane. Nature. 1991;351:320–322. - PubMed
Publication types
MeSH terms
- "V体育官网入口" Actions
- VSports在线直播 - Actions
- V体育安卓版 - Actions
- Actions (V体育ios版)
- Actions (VSports app下载)
- VSports - Actions
- "VSports注册入口" Actions
- Actions (VSports手机版)
- Actions (VSports在线直播)
- V体育安卓版 - Actions
- Actions (V体育官网入口)
- "VSports app下载" Actions
- Actions (VSports在线直播)
- V体育2025版 - Actions
- Actions (VSports注册入口)
- Actions (VSports手机版)
Substances
- VSports注册入口 - Actions
- VSports注册入口 - Actions
- Actions (V体育官网)
- Actions (V体育官网入口)
- "V体育官网入口" Actions
V体育平台登录 - Grants and funding
LinkOut - more resources
VSports - Full Text Sources
Other Literature Sources
Medical
Research Materials
VSports在线直播 - Miscellaneous

